Difference between revisions of "Bcd"
(→Biological materials) |
(→References) |
||
| Line 132: | Line 132: | ||
=References= | =References= | ||
| − | + | ==Reviews== | |
| + | <pubmed>7705101</pubmed> | ||
| + | ==Original publications== | ||
<pubmed>12427936,15241682,10094682,12823818, </pubmed> | <pubmed>12427936,15241682,10094682,12823818, </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:21, 26 December 2011
- Description: valine dehydrogenase, isoleucine dehydrogenase, L-leucine dehydrogenase
| Gene name | bcd |
| Synonyms | bkd, yqiT |
| Essential | no |
| Product | valine dehydrogenase, isoleucine dehydrogenase, L-leucine dehydrogenase |
| Function | utilization of branched-chain keto acids |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis, Ile, Leu, Val | |
| MW, pI | 39 kDa, 4.942 |
| Gene length, protein length | 1092 bp, 364 aa |
| Immediate neighbours | buk, ptb |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 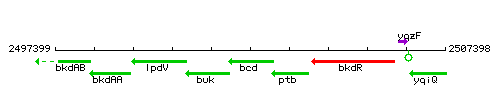
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
BkdR regulon, CodY regulon, SigL regulon
The gene
Basic information
- Locus tag: BSU24080
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-leucine + H2O + NAD+ = 4-methyl-2-oxopentanoate + NH3 + NADH (according to Swiss-Prot)
- Protein family: Glu/Leu/Phe/Val dehydrogenases family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P54531
- KEGG entry: [3]
- E.C. number: 1.4.1.9
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- a bcd mutant and a bcd ybgE ywaA triple mutant are available in Linc Sonenshein's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications