Difference between revisions of "LevD"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || fructose uptake and phosphorylation | |style="background:#ABCDEF;" align="center"|'''Function''' || fructose uptake and phosphorylation | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/carbohydrate_metabolic_pathways.html Sugar catabolism]''' | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 16 kDa, 4.479 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 16 kDa, 4.479 | ||
Revision as of 11:52, 11 June 2009
- Description: fructose-specific phosphotransferase system, EIIA component
| Gene name | levD |
| Synonyms | sacL |
| Essential | no |
| Product | fructose-specific phosphotransferase system, EIIA component |
| Function | fructose uptake and phosphorylation |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism | |
| MW, pI | 16 kDa, 4.479 |
| Gene length, protein length | 438 bp, 146 aa |
| Immediate neighbours | levE, levR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 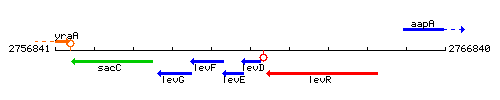
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU27070
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein EIIA N(pi)-phospho-L-histidine + protein EIIB = protein EIIA + protein EIIB N(pi)-phospho-L-histidine/cysteine (according to Swiss-Prot)
- Protein family: PTS permease, mannose permease (Man) family PubMed
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- Swiss prot entry: P26379
- KEGG entry: [2]
- E.C. number: 2.7.1.69
Additional information
Expression and regulation
- Regulation: repressed by glucose (CcpA) , carbon catabolite repression, induction by fructose (LevR)
- Regulatory mechanism: CcpA: transcription repression, catabolite repression: transcription repression by CcpA, transcription activator LevR is less active in the presence of glucose
induction: transcription activation by LevR
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Reizer et al. (1999) Novel phosphotransferase system genes revealed by genome analysis - the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology 145: 3419-3429 PubMed
- Martin-Verstraete, I., Débarbouillé, M., Klier, A., and Rapoport, G. (1990) Levanase operon of Bacillus subtilis includes a fructose-specific phosphotransferase system regulating the expression of the operon. J Mol Biol 214: 657-671. PubMed
- Martin-Verstraete, I. M. Débarbouillé, A. Klier, and G. Rapoport. 1992. Mutagenesis of the Bacillus subtilis ˝-12, -24˝ promoter of the levanase operon and evidence for the existence of an upstream activating sequence. J. Mol. Biol. 226: 85-99. PubMed
- Stülke, J., Martin-Verstraete, I., Charrier, V., Klier, A., Deutscher, J. & Rapoport, G. (1995) The HPr protein of the phosphotransferase system links induction and catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177: 6928-6936. PubMed
- Martin-Verstraete, I., Stülke, J., Klier, A. & Rapoport, G. (1995) Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177: 6919-6927. PubMed
- Martin-Verstraete, I., Charrier, V., Stülke, J., Galinier, A., Erni, B., Rapoport, G., & Deutscher, J. (1998) Antagonistic effects of dual PTS catalyzed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol. Microbiol. 28: 293-303. PubMed
- Charrier V, Deutscher J, Martin-Verstraete I (1997b) Protein phosphorylation chain of a Bacillus subtilis fructose-specific phosphotransferase system and its participation in regulation of the expression of the lev operon. Biochemistry 36:1163-1172. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed