IolG
- Description: inositol 2-dehydrogenase
| Gene name | iolG |
| Synonyms | idh, iol |
| Essential | no |
| Product | inositol 2-dehydrogenase |
| Function | myo-inositol catabolism |
| Gene expression levels in SubtiExpress: iolG | |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism | |
| MW, pI | 38 kDa, 4.865 |
| Gene length, protein length | 1032 bp, 344 aa |
| Immediate neighbours | iolH, iolF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 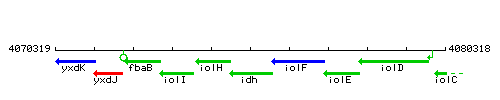
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed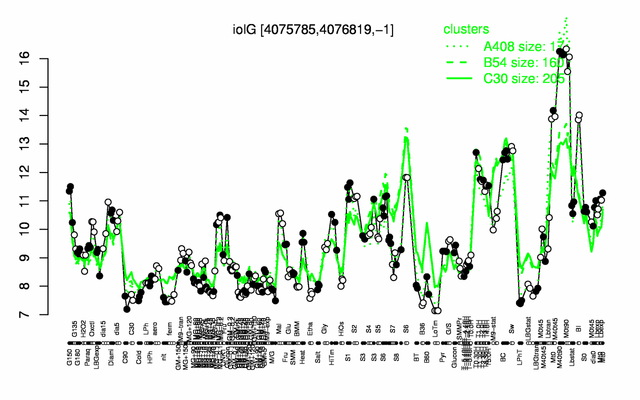
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU39700
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Myo-inositol + NAD+ = 2,4,6/3,5-pentahydroxycyclohexanone + NADH (according to Swiss-Prot)
- Protein family: gfo/idh/mocA family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Interactions:
- the protein forms a tetramer PubMed
Database entries
- UniProt: P26935
- KEGG entry: [3]
- E.C. number: 1.1.1.18
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Yasutaro Fujita, University of Fukuyama, Japan
- Ken-ichi Yoshida, Kobe University, Japan
Your additional remarks
References
Kosei Tanaka, Shintaro Tajima, Shinji Takenaka, Ken-ichi Yoshida
An improved Bacillus subtilis cell factory for producing scyllo-inositol, a promising therapeutic agent for Alzheimer's disease.
Microb Cell Fact: 2013, 12;124
[PubMed:24325193]
[WorldCat.org]
[DOI]
(I e)
Hongyan Zheng, Drew Bertwistle, David A R Sanders, David R J Palmer
Converting NAD-specific inositol dehydrogenase to an efficient NADP-selective catalyst, with a surprising twist.
Biochemistry: 2013, 52(34);5876-83
[PubMed:23952058]
[WorldCat.org]
[DOI]
(I p)
Karin E van Straaten, Hongyan Zheng, David R J Palmer, David A R Sanders
Structural investigation of myo-inositol dehydrogenase from Bacillus subtilis: implications for catalytic mechanism and inositol dehydrogenase subfamily classification.
Biochem J: 2010, 432(2);237-47
[PubMed:20809899]
[WorldCat.org]
[DOI]
(I p)
Ken-ichi Yoshida, Masanori Yamaguchi, Tetsuro Morinaga, Masaki Kinehara, Maya Ikeuchi, Hitoshi Ashida, Yasutaro Fujita
myo-Inositol catabolism in Bacillus subtilis.
J Biol Chem: 2008, 283(16);10415-24
[PubMed:18310071]
[WorldCat.org]
[DOI]
(P p)
K I Yoshida, T Shibayama, D Aoyama, Y Fujita
Interaction of a repressor and its binding sites for regulation of the Bacillus subtilis iol divergon.
J Mol Biol: 1999, 285(3);917-29
[PubMed:9887260]
[WorldCat.org]
[DOI]
(P p)
K I Yoshida, D Aoyama, I Ishio, T Shibayama, Y Fujita
Organization and transcription of the myo-inositol operon, iol, of Bacillus subtilis.
J Bacteriol: 1997, 179(14);4591-8
[PubMed:9226270]
[WorldCat.org]
[DOI]
(P p)
Y Fujita, K Shindo, Y Miwa, K Yoshida
Bacillus subtilis inositol dehydrogenase-encoding gene (idh): sequence and expression in Escherichia coli.
Gene: 1991, 108(1);121-5
[PubMed:1761221]
[WorldCat.org]
[DOI]
(P p)
J Nihashi, Y Fujita
Catabolite repression of inositol dehydrogenase and gluconate kinase syntheses in Bacillus subtilis.
Biochim Biophys Acta: 1984, 798(1);88-95
[PubMed:6322857]
[WorldCat.org]
[DOI]
(P p)
R Ramaley, Y Fujita, E Freese
Purification and properties of Bacillus subtilis inositol dehydrogenase.
J Biol Chem: 1979, 254(16);7684-90
[PubMed:112095]
[WorldCat.org]
(P p)