SpoIIAA
- Description: anti-anti-SigF
| Gene name | spoIIAA |
| Synonyms | |
| Essential | no |
| Product | anti-anti-SigF |
| Function | control of SigF activity |
| Gene expression levels in SubtiExpress: spoIIAA | |
| Interactions involving this protein in SubtInteract: SpoIIAA | |
| Metabolic function and regulation of this protein in SubtiPathways: spoIIAA | |
| MW, pI | 12 kDa, 5.855 |
| Gene length, protein length | 351 bp, 117 aa |
| Immediate neighbours | spoIIAB, dacF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 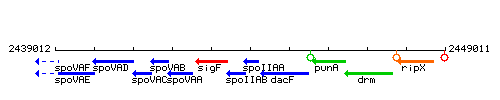
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed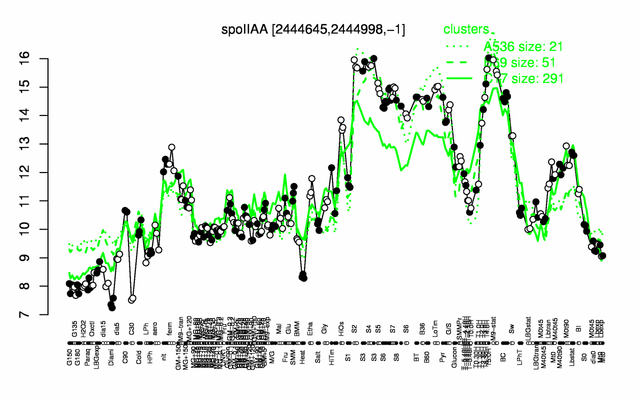
| |
Contents
Categories containing this gene/protein
sigma factors and their control, sporulation proteins, phosphoproteins
This gene is a member of the following regulons
AbrB regulon, SigF regulon, SigG regulon, SigH regulon, SinR regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU23470
Phenotypes of a mutant
Database entries
- BsubCyc: BSU23470
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: anti-sigma-factor antagonist family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU23470
- UniProt: P10727
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation: repressed by glucose (5.2-fold) PubMed, dacF: expressed during sporulation, spoIIAA: expressed early during sporulation
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 388 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 237 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 3471 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Tony Wilkinson, York University, U.K. homepage
- Charles Moran, Emory University, NC, USA homepage
Your additional remarks
References
Modeling of SigF activation
Original Publications
Onuma Chumsakul, Hiroki Takahashi, Taku Oshima, Takahiro Hishimoto, Shigehiko Kanaya, Naotake Ogasawara, Shu Ishikawa
Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation.
Nucleic Acids Res: 2011, 39(2);414-28
[PubMed:20817675]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Oleg A Igoshin, Chester W Price, Michael A Savageau
Signalling network with a bistable hysteretic switch controls developmental activation of the sigma transcription factor in Bacillus subtilis.
Mol Microbiol: 2006, 61(1);165-84
[PubMed:16824103]
[WorldCat.org]
[DOI]
(P p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Masaya Fujita, José Eduardo González-Pastor, Richard Losick
High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis.
J Bacteriol: 2005, 187(4);1357-68
[PubMed:15687200]
[WorldCat.org]
[DOI]
(P p)
Joanna Clarkson, Iain D Campbell, Michael D Yudkin
Efficient regulation of sigmaF, the first sporulation-specific sigma factor in B.subtilis.
J Mol Biol: 2004, 342(4);1187-95
[PubMed:15351644]
[WorldCat.org]
[DOI]
(P p)
Joanna Clarkson, Iain D Campbell, Michael D Yudkin
Physical evidence for the induced release of the Bacillus subtilis transcription factor, sigma(F), from its inhibitory complex.
J Mol Biol: 2004, 340(2);203-9
[PubMed:15201047]
[WorldCat.org]
[DOI]
(P p)
Joanna Clarkson, Jwu-Ching Shu, David A Harris, Iain D Campbell, Michael D Yudkin
Fluorescence and kinetic analysis of the SpoIIAB phosphorylation reaction, a key regulator of sporulation in Bacillus subtilis.
Biochemistry: 2004, 43(11);3120-8
[PubMed:15023063]
[WorldCat.org]
[DOI]
(P p)
Karen Carniol, Patrick Eichenberger, Richard Losick
A threshold mechanism governing activation of the developmental regulatory protein sigma F in Bacillus subtilis.
J Biol Chem: 2004, 279(15);14860-70
[PubMed:14744853]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
Margaret S Ho, Karen Carniol, Richard Losick
Evidence in support of a docking model for the release of the transcription factor sigma F from the antisigma factor SpoIIAB in Bacillus subtilis.
J Biol Chem: 2003, 278(23);20898-905
[PubMed:12676949]
[WorldCat.org]
[DOI]
(P p)
J Clarkson, I D Campbell, M D Yudkin
NMR studies of the interactions of SpoIIAA with its partner proteins that regulate sporulation in Bacillus subtilis.
J Mol Biol: 2001, 314(3);359-64
[PubMed:11846550]
[WorldCat.org]
[DOI]
(P p)
Q Pan, D A Garsin, R Losick
Self-reinforcing activation of a cell-specific transcription factor by proteolysis of an anti-sigma factor in B. subtilis.
Mol Cell: 2001, 8(4);873-83
[PubMed:11684022]
[WorldCat.org]
[DOI]
(P p)
C S Lee, J Clarkson, J C Shu, I D Campbell, M D Yudkin
Bacillus subtilis mutations that alter the pathway of phosphorylation of the anti-anti-sigmaF factor SpoIIAA lead to a Spo- phenotype.
Mol Microbiol: 2001, 40(1);9-19
[PubMed:11298272]
[WorldCat.org]
[DOI]
(P p)
A Feucht, R A Daniel, J Errington
Characterization of a morphological checkpoint coupling cell-specific transcription to septation in Bacillus subtilis.
Mol Microbiol: 1999, 33(5);1015-26
[PubMed:10476035]
[WorldCat.org]
[DOI]
(P p)
N King, O Dreesen, P Stragier, K Pogliano, R Losick
Septation, dephosphorylation, and the activation of sigmaF during sporulation in Bacillus subtilis.
Genes Dev: 1999, 13(9);1156-67
[PubMed:10323866]
[WorldCat.org]
[DOI]
(P p)
N Frandsen, I Barák, C Karmazyn-Campelli, P Stragier
Transient gene asymmetry during sporulation and establishment of cell specificity in Bacillus subtilis.
Genes Dev: 1999, 13(4);394-9
[PubMed:10049355]
[WorldCat.org]
[DOI]
(P p)
D A Garsin, L Duncan, D M Paskowitz, R Losick
The kinase activity of the antisigma factor SpoIIAB is required for activation as well as inhibition of transcription factor sigmaF during sporulation in Bacillus subtilis.
J Mol Biol: 1998, 284(3);569-78
[PubMed:9826499]
[WorldCat.org]
[DOI]
(P p)
D A Garsin, D M Paskowitz, L Duncan, R Losick
Evidence for common sites of contact between the antisigma factor SpoIIAB and its partners SpoIIAA and the developmental transcription factor sigmaF in Bacillus subtilis.
J Mol Biol: 1998, 284(3);557-68
[PubMed:9826498]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, L J Wu, J Errington
Establishment of prespore-specific gene expression in Bacillus subtilis: localization of SpoIIE phosphatase and initiation of compartment-specific proteolysis.
J Bacteriol: 1998, 180(13);3276-84
[PubMed:9642177]
[WorldCat.org]
[DOI]
(P p)
M Lord, T Magnin, M D Yudkin
Protein conformational change and nucleotide binding involved in regulation of sigmaF in Bacillus subtilis.
J Bacteriol: 1996, 178(23);6730-5
[PubMed:8955289]
[WorldCat.org]
[DOI]
(P p)
S M Najafi, D A Harris, M D Yudkin
The SpoIIAA protein of Bacillus subtilis has GTP-binding properties.
J Bacteriol: 1996, 178(22);6632-4
[PubMed:8932322]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, T Magnin, J Errington
Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor sigmaF of Bacillus subtilis.
Genes Cells: 1996, 1(10);881-94
[PubMed:9077448]
[WorldCat.org]
[DOI]
(P p)
S Alper, A Dufour, D A Garsin, L Duncan, R Losick
Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis.
J Mol Biol: 1996, 260(2);165-77
[PubMed:8764398]
[WorldCat.org]
[DOI]
(P p)
L Duncan, S Alper, R Losick
SpoIIAA governs the release of the cell-type specific transcription factor sigma F from its anti-sigma factor SpoIIAB.
J Mol Biol: 1996, 260(2);147-64
[PubMed:8764397]
[WorldCat.org]
[DOI]
(P p)
F Arigoni, L Duncan, S Alper, R Losick, P Stragier
SpoIIE governs the phosphorylation state of a protein regulating transcription factor sigma F during sporulation in Bacillus subtilis.
Proc Natl Acad Sci U S A: 1996, 93(8);3238-42
[PubMed:8622920]
[WorldCat.org]
[DOI]
(P p)
A Feucht, T Magnin, M D Yudkin, J Errington
Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis.
Genes Dev: 1996, 10(7);794-803
[PubMed:8846916]
[WorldCat.org]
[DOI]
(P p)
T Magnin, M Lord, J Errington, M D Yudkin
Establishing differential gene expression in sporulating Bacillus subtilis: phosphorylation of SpoIIAA (anti-anti-sigmaF) alters its conformation and prevents formation of a SpoIIAA/SpoIIAB/ADP complex.
Mol Microbiol: 1996, 19(4);901-7
[PubMed:8820658]
[WorldCat.org]
[DOI]
(P p)
L Duncan, S Alper, F Arigoni, R Losick, P Stragier
Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division.
Science: 1995, 270(5236);641-4
[PubMed:7570023]
[WorldCat.org]
[DOI]
(P p)
S Alper, L Duncan, R Losick
An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis.
Cell: 1994, 77(2);195-205
[PubMed:8168129]
[WorldCat.org]
[DOI]
(P p)
K T Min, C M Hilditch, B Diederich, J Errington, M D Yudkin
Sigma F, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase.
Cell: 1993, 74(4);735-42
[PubMed:8358793]
[WorldCat.org]
[DOI]
(P p)
T Bird, D Burbulys, J J Wu, M A Strauch, J A Hoch, G B Spiegelman
The effect of supercoiling on the in vitro transcription of the spoIIA operon from Bacillus subtilis.
Biochimie: 1992, 74(7-8);627-34
[PubMed:1391042]
[WorldCat.org]
[DOI]
(P p)
K York, T J Kenney, S Satola, C P Moran, H Poth, P Youngman
Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE.
J Bacteriol: 1992, 174(8);2648-58
[PubMed:1556084]
[WorldCat.org]
[DOI]
(P p)
P Margolis, A Driks, R Losick
Establishment of cell type by compartmentalized activation of a transcription factor.
Science: 1991, 254(5031);562-5
[PubMed:1948031]
[WorldCat.org]
[DOI]
(P p)
R Schmidt, P Margolis, L Duncan, R Coppolecchia, C P Moran, R Losick
Control of developmental transcription factor sigma F by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis.
Proc Natl Acad Sci U S A: 1990, 87(23);9221-5
[PubMed:2123551]
[WorldCat.org]
[DOI]
(P p)
J Errington, J Mandelstam
Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis.
J Gen Microbiol: 1986, 132(11);2967-76
[PubMed:3114419]
[WorldCat.org]
[DOI]
(P p)