Hag
Revision as of 14:08, 17 April 2014 by 134.76.70.252 (talk)
- Description: flagellin protein, about 20,000 subunits make up one flagellum
| Gene name | hag |
| Synonyms | |
| Essential | no |
| Product | flagellin protein |
| Function | motility and chemotaxis |
| Gene expression levels in SubtiExpress: hag | |
| Interactions involving this protein in SubtInteract: Hag | |
| Metabolic function and regulation of this protein in SubtiPathways: Hag | |
| MW, pI | 32 kDa, 4.782 |
| Gene length, protein length | 912 bp, 304 aa |
| Immediate neighbours | yvyC, csrA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 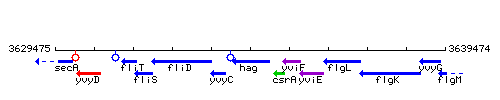
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
motility and chemotaxis, most abundant proteins
This gene is a member of the following regulons
CodY regulon, CsrA regulon, ScoC regulon, SigD regulon
The gene
Basic information
- Locus tag: BSU35360
Phenotypes of a mutant
No swarming motility on B medium. PubMed
Database entries
- BsubCyc: BSU35360
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: bacterial flagellin family (according to Swiss-Prot)
- Paralogous protein(s): YvzB (C-terminal domain of Hag)
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU35360
- Structure: 3A5X (from Salmonella typhimurium, 42% identity)
- UniProt: P02968
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
- the hag gene is strongly overexpressed in a ymdB mutant (loss of bistable gene expression) PubMed
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 150241 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 280953 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 9552 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 11147 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 24114 PubMed
Biological materials
- Mutant: GP901 (aphA3), GP902 (tet) PubMed, both available in Jörg Stülke's lab
- Expression vector:
- expression in B. subtilis, in pBQ200: pGP1089, available in Jörg Stülke's lab
- lacZ fusion: pGP1035 (in pAC6), pGP755 (in pAC7), there is also a series of promoter deletion variants in pAC6 and pAC7 PubMed, all available in Jörg Stülke's lab
- GFP fusion: BP494 (bglS:: (hag-promoter-cfp-aphA3)), BP496 (amyE:: (hag-promoter-iyfp-cat)), available in Jörg Stülke's lab
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Tony Romeo, Christopher A Vakulskas, Paul Babitzke
Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems.
Environ Microbiol: 2013, 15(2);313-24
[PubMed:22672726]
[WorldCat.org]
[DOI]
(I p)
Original Publications