Spo0F
- Description: phosphotransferase of the sporulation initiation phosphorelay
| Gene name | spo0F |
| Synonyms | |
| Essential | no |
| Product | phosphotransferase of the sporulation initiation phosphorelay |
| Function | initiation of sporulation |
| Function and regulation of this protein in SubtiPathways: Phosphorelay | |
| MW, pI | 14 kDa, 4.697 |
| Gene length, protein length | 372 bp, 124 aa |
| Immediate neighbours | fbaA, ywjG |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 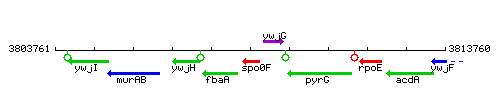
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU37130
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions: Spo0F-Spo0B, RapA-Spo0F, RapB-Spo0F PubMed, RapE-Spo0F, RapH-Spo0F PubMed, KinA-Spo0F, KinB-Spo0F, KinC-Spo0F, KinD-Spo0F, KinE-Spo0F
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P06628
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: spo0F PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Prahathees Eswaramoorthy, Jeffrey Dinh, Daniel Duan, Oleg A Igoshin, Masaya Fujita
Single-cell measurement of the levels and distributions of the phosphorelay components in a population of sporulating Bacillus subtilis cells.
Microbiology (Reading): 2010, 156(Pt 8);2294-2304
[PubMed:20413551]
[WorldCat.org]
[DOI]
(I p)
Wiep Klaas Smits, Cristina Bongiorni, Jan-Willem Veening, Leendert W Hamoen, Oscar P Kuipers, Marta Perego
Temporal separation of distinct differentiation pathways by a dual specificity Rap-Phr system in Bacillus subtilis.
Mol Microbiol: 2007, 65(1);103-20
[PubMed:17581123]
[WorldCat.org]
[DOI]
(P p)
Patrick D McLaughlin, Benjamin G Bobay, Erin J Regel, Richele J Thompson, James A Hoch, John Cavanagh
Predominantly buried residues in the response regulator Spo0F influence specific sensor kinase recognition.
FEBS Lett: 2007, 581(7);1425-9
[PubMed:17350627]
[WorldCat.org]
[DOI]
(P p)
Kottayil I Varughese, Igor Tsigelny, Haiyan Zhao
The crystal structure of beryllofluoride Spo0F in complex with the phosphotransferase Spo0B represents a phosphotransfer pretransition state.
J Bacteriol: 2006, 188(13);4970-7
[PubMed:16788205]
[WorldCat.org]
[DOI]
(P p)
Douglas J Kojetin, Richele J Thompson, Linda M Benson, Stephen Naylor, Jenora Waterman, Keith G Davies, Charles H Opperman, Keith Stephenson, James A Hoch, John Cavanagh
Structural analysis of divalent metals binding to the Bacillus subtilis response regulator Spo0F: the possibility for in vitro metalloregulation in the initiation of sporulation.
Biometals: 2005, 18(5);449-66
[PubMed:16333746]
[WorldCat.org]
[DOI]
(P p)
Sophie J Stephenson, Marta Perego
Interaction surface of the Spo0A response regulator with the Spo0E phosphatase.
Mol Microbiol: 2002, 44(6);1455-67
[PubMed:12067336]
[WorldCat.org]
[DOI]
(P p)
Shu Ishikawa, Leighton Core, Marta Perego
Biochemical characterization of aspartyl phosphate phosphatase interaction with a phosphorylated response regulator and its inhibition by a pentapeptide.
J Biol Chem: 2002, 277(23);20483-9
[PubMed:11923303]
[WorldCat.org]
[DOI]
(P p)
M Jiang, W Shao, M Perego, J A Hoch
Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis.
Mol Microbiol: 2000, 38(3);535-42
[PubMed:11069677]
[WorldCat.org]
[DOI]
(P p)
J Zapf, U Sen, Madhusudan, J A Hoch, K I Varughese
A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction.
Structure: 2000, 8(8);851-62
[PubMed:10997904]
[WorldCat.org]
[DOI]
(P p)
M Jiang, Y L Tzeng, V A Feher, M Perego, J A Hoch
Alanine mutants of the Spo0F response regulator modifying specificity for sensor kinases in sporulation initiation.
Mol Microbiol: 1999, 33(2);389-95
[PubMed:10411754]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)
Y L Tzeng, V A Feher, J Cavanagh, M Perego, J A Hoch
Characterization of interactions between a two-component response regulator, Spo0F, and its phosphatase, RapB.
Biochemistry: 1998, 37(47);16538-45
[PubMed:9843420]
[WorldCat.org]
[DOI]
(P p)
J Zapf, M Madhusudan, C E Grimshaw, J A Hoch, K I Varughese, J M Whiteley
A source of response regulator autophosphatase activity: the critical role of a residue adjacent to the Spo0F autophosphorylation active site.
Biochemistry: 1998, 37(21);7725-32
[PubMed:9601032]
[WorldCat.org]
[DOI]
(P p)
V A Feher, Y L Tzeng, J A Hoch, J Cavanagh
Identification of communication networks in Spo0F: a model for phosphorylation-induced conformational change and implications for activation of multiple domain bacterial response regulators.
FEBS Lett: 1998, 425(1);1-6
[PubMed:9540996]
[WorldCat.org]
[DOI]
(P p)
C E Grimshaw, S Huang, C G Hanstein, M A Strauch, D Burbulys, L Wang, J A Hoch, J M Whiteley
Synergistic kinetic interactions between components of the phosphorelay controlling sporulation in Bacillus subtilis.
Biochemistry: 1998, 37(5);1365-75
[PubMed:9477965]
[WorldCat.org]
[DOI]
(P p)
M Madhusudan, J Zapf, J A Hoch, J M Whiteley, N H Xuong, K I Varughese
A response regulatory protein with the site of phosphorylation blocked by an arginine interaction: crystal structure of Spo0F from Bacillus subtilis.
Biochemistry: 1997, 36(42);12739-45
[PubMed:9335530]
[WorldCat.org]
[DOI]
(P p)
Y L Tzeng, J A Hoch
Molecular recognition in signal transduction: the interaction surfaces of the Spo0F response regulator with its cognate phosphorelay proteins revealed by alanine scanning mutagenesis.
J Mol Biol: 1997, 272(2);200-12
[PubMed:9299348]
[WorldCat.org]
[DOI]
(P p)
V A Feher, J W Zapf, J A Hoch, J M Whiteley, L P McIntosh, M Rance, N J Skelton, F W Dahlquist, J Cavanagh
High-resolution NMR structure and backbone dynamics of the Bacillus subtilis response regulator, Spo0F: implications for phosphorylation and molecular recognition.
Biochemistry: 1997, 36(33);10015-25
[PubMed:9254596]
[WorldCat.org]
[DOI]
(P p)
Madhusudan, J Zapf, J M Whiteley, J A Hoch, N H Xuong, K I Varughese
Crystal structure of a phosphatase-resistant mutant of sporulation response regulator Spo0F from Bacillus subtilis.
Structure: 1996, 4(6);679-90
[PubMed:8805550]
[WorldCat.org]
[DOI]
(P p)
Madhusudan, J Zapf, J M Whiteley, J A Hoch, N H Xuong, K I Varughese
Crystallization and preliminary X-ray analysis of a Y13S mutant of Spo0F from Bacillus subtilis.
Acta Crystallogr D Biol Crystallogr: 1996, 52(Pt 3);589-90
[PubMed:15299688]
[WorldCat.org]
[DOI]
(P p)
J W Zapf, J A Hoch, J M Whiteley
A phosphotransferase activity of the Bacillus subtilis sporulation protein Spo0F that employs phosphoramidate substrates.
Biochemistry: 1996, 35(9);2926-33
[PubMed:8608130]
[WorldCat.org]
[DOI]
(P p)
M Perego, P Glaser, J A Hoch
Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis.
Mol Microbiol: 1996, 19(6);1151-7
[PubMed:8730857]
[WorldCat.org]
[DOI]
(P p)
M Perego, J A Hoch
Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis.
Proc Natl Acad Sci U S A: 1996, 93(4);1549-53
[PubMed:8643670]
[WorldCat.org]
[DOI]
(P p)
K A Trach, J A Hoch
Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway.
Mol Microbiol: 1993, 8(1);69-79
[PubMed:8497199]
[WorldCat.org]
[DOI]
(P p)
M A Strauch, J J Wu, R H Jonas, J A Hoch
A positive feedback loop controls transcription of the spoOF gene, a component of the sporulation phosphorelay in Bacillus subtilis.
Mol Microbiol: 1993, 7(6);967-74
[PubMed:8483422]
[WorldCat.org]
[DOI]
(P p)
M Predich, G Nair, I Smith
Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by the RNA polymerase containing sigma H.
J Bacteriol: 1992, 174(9);2771-8
[PubMed:1569009]
[WorldCat.org]
[DOI]
(P p)
D Burbulys, K A Trach, J A Hoch
Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay.
Cell: 1991, 64(3);545-52
[PubMed:1846779]
[WorldCat.org]
[DOI]
(P p)
U Bai, M Lewandoski, E Dubnau, I Smith
Temporal regulation of the Bacillus subtilis early sporulation gene spo0F.
J Bacteriol: 1990, 172(9);5432-9
[PubMed:2118512]
[WorldCat.org]
[DOI]
(P p)
M Perego, S P Cole, D Burbulys, K Trach, J A Hoch
Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis.
J Bacteriol: 1989, 171(11);6187-96
[PubMed:2509430]
[WorldCat.org]
[DOI]
(P p)
K Trach, J W Chapman, P Piggot, D LeCoq, J A Hoch
Complete sequence and transcriptional analysis of the spo0F region of the Bacillus subtilis chromosome.
J Bacteriol: 1988, 170(9);4194-208
[PubMed:2457578]
[WorldCat.org]
[DOI]
(P p)
K A Trach, J W Chapman, P J Piggot, J A Hoch
Deduced product of the stage 0 sporulation gene spo0F shares homology with the Spo0A, OmpR, and SfrA proteins.
Proc Natl Acad Sci U S A: 1985, 82(21);7260-4
[PubMed:2997779]
[WorldCat.org]
[DOI]
(P p)