ArgH
Revision as of 11:00, 14 May 2013 by 134.76.70.252 (talk)
- Description: argininosuccinate lyase
| Gene name | argH |
| Synonyms | |
| Essential | no |
| Product | argininosuccinate lyase |
| Function | biosynthesis of arginine |
| Gene expression levels in SubtiExpress: argH | |
| Metabolic function and regulation of this protein in SubtiPathways: Ammonium/ glutamate | |
| MW, pI | 51 kDa, 4.859 |
| Gene length, protein length | 1383 bp, 461 aa |
| Immediate neighbours | ytzD, argG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 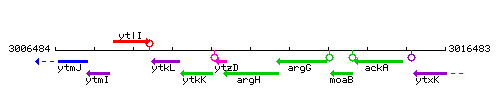
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed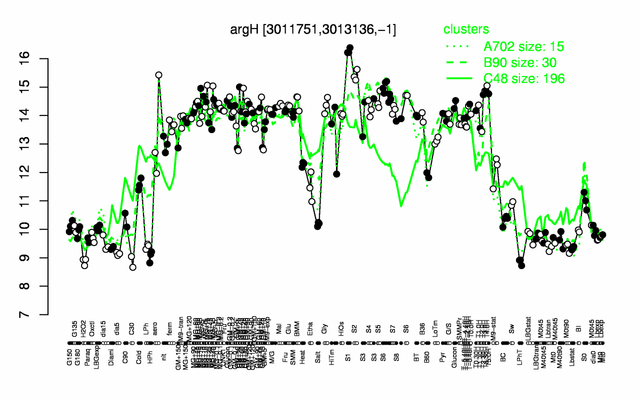
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29440
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
- A mutation was found in this gene after evolution under relaxed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2-(N(omega)-L-arginino)succinate = fumarate + L-arginine (according to Swiss-Prot)
- Protein family: Argininosuccinate lyase subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: O34858
- KEGG entry: [2]
- E.C. number: 4.3.2.1
Additional information
Expression and regulation
- Sigma factor:
- Regulatory mechanism:
- AhrC: transcription repression
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References