TrxA
- Description: antioxidative action by facilitating the reduction of other proteins by cysteine thiol-disulfide exchange
| Gene name | trxA |
| Synonyms | trx |
| Essential | yes PubMed |
| Product | thioredoxin |
| Function | protection of proteins against oxidative damage |
| Gene expression levels in SubtiExpress: trxA | |
| Interactions involving this protein in SubtInteract: TrxA | |
| MW, pI | 11 kDa, 4.308 |
| Gene length, protein length | 312 bp, 104 aa |
| Immediate neighbours | uvrC, abf2 |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 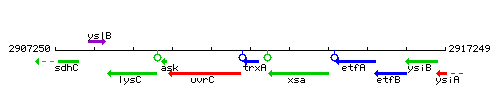
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed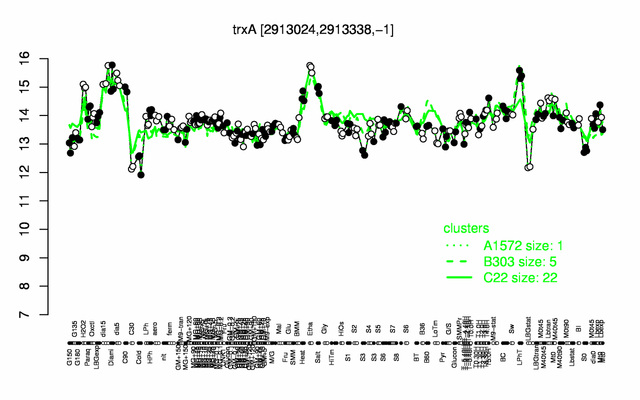
| |
Contents
Categories containing this gene/protein
electron transport/ other, general stress proteins (controlled by SigB), resistance against oxidative and electrophile stress, essential genes
This gene is a member of the following regulons
CtsR regulon, SigB regulon, Spx regulon
The gene
Basic information
- Locus tag: BSU28500
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: thioredoxin family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm PubMed
Database entries
- UniProt: P14949
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: trxA PubMed
- Regulation:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Michiko M Nakano, Ann Lin, Cole S Zuber, Kate J Newberry, Richard G Brennan, Peter Zuber
Promoter recognition by a complex of Spx and the C-terminal domain of the RNA polymerase alpha subunit.
PLoS One: 2010, 5(1);e8664
[PubMed:20084284]
[WorldCat.org]
[DOI]
(I e)
Dindo Y Reyes, Peter Zuber
Activation of transcription initiation by Spx: formation of transcription complex and identification of a Cis-acting element required for transcriptional activation.
Mol Microbiol: 2008, 69(3);765-79
[PubMed:18687074]
[WorldCat.org]
[DOI]
(I p)
Jörg Mostertz, Falko Hochgräfe, Britta Jürgen, Thomas Schweder, Michael Hecker
The role of thioredoxin TrxA in Bacillus subtilis: a proteomics and transcriptomics approach.
Proteomics: 2008, 8(13);2676-90
[PubMed:18601268]
[WorldCat.org]
[DOI]
(I p)
Mirja Carlsson Möller, Lars Hederstedt
Extracytoplasmic processes impaired by inactivation of trxA (thioredoxin gene) in Bacillus subtilis.
J Bacteriol: 2008, 190(13);4660-5
[PubMed:18456801]
[WorldCat.org]
[DOI]
(I p)
Thijs R H M Kouwen, Annemieke van der Goot, Ronald Dorenbos, Theresa Winter, Haike Antelmann, Marie-Claire Plaisier, Wim J Quax, January Maarten van Dijl, Jean-Yves F Dubois
Thiol-disulphide oxidoreductase modules in the low-GC Gram-positive bacteria.
Mol Microbiol: 2007, 64(4);984-99
[PubMed:17501922]
[WorldCat.org]
[DOI]
(P p)
You Li, Yunfei Hu, Xinxin Zhang, Huimin Xu, Ewen Lescop, Bin Xia, Changwen Jin
Conformational fluctuations coupled to the thiol-disulfide transfer between thioredoxin and arsenate reductase in Bacillus subtilis.
J Biol Chem: 2007, 282(15);11078-83
[PubMed:17303556]
[WorldCat.org]
[DOI]
(P p)
Wiep Klaas Smits, Jean-Yves F Dubois, Sierd Bron, Jan Maarten van Dijl, Oscar P Kuipers
Tricksy business: transcriptome analysis reveals the involvement of thioredoxin A in redox homeostasis, oxidative stress, sulfur metabolism, and cellular differentiation in Bacillus subtilis.
J Bacteriol: 2005, 187(12);3921-30
[PubMed:15937154]
[WorldCat.org]
[DOI]
(P p)
Shunji Nakano, Michiko M Nakano, Ying Zhang, Montira Leelakriangsak, Peter Zuber
A regulatory protein that interferes with activator-stimulated transcription in bacteria.
Proc Natl Acad Sci U S A: 2003, 100(7);4233-8
[PubMed:12642660]
[WorldCat.org]
[DOI]
(P p)
A Petersohn, M Brigulla, S Haas, J D Hoheisel, U Völker, M Hecker
Global analysis of the general stress response of Bacillus subtilis.
J Bacteriol: 2001, 183(19);5617-31
[PubMed:11544224]
[WorldCat.org]
[DOI]
(P p)
E Krüger, M Hecker
The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes.
J Bacteriol: 1998, 180(24);6681-8
[PubMed:9852015]
[WorldCat.org]
[DOI]
(P p)
C Scharf, S Riethdorf, H Ernst, S Engelmann, U Völker, M Hecker
Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis.
J Bacteriol: 1998, 180(7);1869-77
[PubMed:9537387]
[WorldCat.org]
[DOI]
(P p)