SinI
| Gene name | sinI |
| Synonyms | |
| Essential | no |
| Product | antagonist of SinR |
| Function | control of biofilm formation |
| Interactions involving this protein in SubtInteract: SinI | |
| Regulation of this protein in SubtiPathways: Biofilm | |
| MW, pI | 6 kDa, 6.333 |
| Gene length, protein length | 171 bp, 57 aa |
| Immediate neighbours | yqhG, sinR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 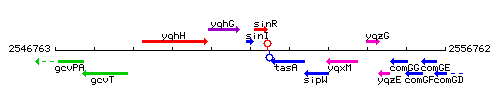
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
transcription factors and their control, transition state regulators, biofilm formation
This gene is a member of the following regulons
AbrB regulon, ScoC regulon, Spo0A regulon
The gene
Basic information
- Locus tag: BSU24600
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s): SlrA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P23308
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- FLAG-tag construct (C-term): GP935 (kan), available in Stülke lab
- Antibody:
Labs working on this gene/ protein
Your additional remarks
References
Reviews
Modelling of the SinI/SinR switch
Original publications
Additonal publications: PubMed
Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011 81(6): 1459-1473. PubMed:21815947