Difference between revisions of "RibE"
| Line 62: | Line 62: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 80: | Line 77: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 137: | Line 134: | ||
=References= | =References= | ||
| − | <pubmed>12456892,15808508,11377200 , 8159171, 7934830 7473709 23270261 </pubmed> | + | <pubmed>12456892,15808508,11377200 , 8159171, 7934830 7473709 23270261 24442413 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:58, 21 January 2014
- Description: riboflavin synthase (alpha subunit)
| Gene name | ribE |
| Synonyms | ribB |
| Essential | no |
| Product | riboflavin synthase (alpha subunit) |
| Function | riboflavin biosynthesis |
| Gene expression levels in SubtiExpress: ribE | |
| Interactions involving this protein in SubtInteract: RibE | |
| Metabolic function and regulation of this protein in SubtiPathways: ribE | |
| MW, pI | 23 kDa, 5.845 |
| Gene length, protein length | 645 bp, 215 aa |
| Immediate neighbours | ribA, ribD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 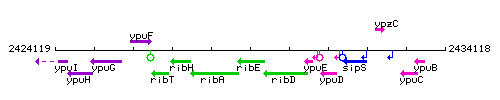
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU23270
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 6,7-dimethyl-8-(1-D-ribityl)lumazine = riboflavin + 4-(1-D-ribitylamino)-5-amino-2,6-dihydroxypyrimidine (according to Swiss-Prot)
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure: 1I8D (from E. coli, 36% identity, 59% similarity) PubMed, , 1RVV (the RibE)3-(RibH)60 lumazine synthase/riboflavin synthase complex) PubMed
- UniProt: P16440
- KEGG entry: [3]
- E.C. number: 2.5.1.9
Additional information
Expression and regulation
- Regulatory mechanism: FMN-box: riboswitch, mediates termination/ antitermination control of the operon, in the absence of FMN: antitermination, in the presence of FMN: termination PubMed
- Additional information:
Biological materials
- Mutant: GP203 (erm), available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Markus Birkenmeier, Susanne Neumann, Thorsten Röder
Kinetic modeling of riboflavin biosynthesis in Bacillus subtilis under production conditions.
Biotechnol Lett: 2014, 36(5);919-28
[PubMed:24442413]
[WorldCat.org]
[DOI]
(I p)
S A Skliarova, R A Kreneva, D A Perumov, A S Mironov
[The characterization of internal promoters in the Bacillus subtilis riboflavin biosynthesis operon].
Genetika: 2012, 48(10);1133-41
[PubMed:23270261]
[WorldCat.org]
(P p)
J Kenneth Wickiser, Wade C Winkler, Ronald R Breaker, Donald M Crothers
The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch.
Mol Cell: 2005, 18(1);49-60
[PubMed:15808508]
[WorldCat.org]
[DOI]
(P p)
Wade C Winkler, Smadar Cohen-Chalamish, Ronald R Breaker
An mRNA structure that controls gene expression by binding FMN.
Proc Natl Acad Sci U S A: 2002, 99(25);15908-13
[PubMed:12456892]
[WorldCat.org]
[DOI]
(P p)
D I Liao, Z Wawrzak, J C Calabrese, P V Viitanen, D B Jordan
Crystal structure of riboflavin synthase.
Structure: 2001, 9(5);399-408
[PubMed:11377200]
[WorldCat.org]
[DOI]
(P p)
K Ritsert, R Huber, D Turk, R Ladenstein, K Schmidt-Bäse, A Bacher
Studies on the lumazine synthase/riboflavin synthase complex of Bacillus subtilis: crystal structure analysis of reconstituted, icosahedral beta-subunit capsids with bound substrate analogue inhibitor at 2.4 A resolution.
J Mol Biol: 1995, 253(1);151-67
[PubMed:7473709]
[WorldCat.org]
[DOI]
(P p)
V N Mironov, A S Kraev, M L Chikindas, B K Chernov, A I Stepanov, K G Skryabin
Functional organization of the riboflavin biosynthesis operon from Bacillus subtilis SHgw.
Mol Gen Genet: 1994, 242(2);201-8
[PubMed:8159171]
[WorldCat.org]
[DOI]
(P p)
V Azevedo, A Sorokin, S D Ehrlich, P Serror
The transcriptional organization of the Bacillus subtilis 168 chromosome region between the spoVAF and serA genetic loci.
Mol Microbiol: 1993, 10(2);397-405
[PubMed:7934830]
[WorldCat.org]
[DOI]
(P p)