Difference between revisions of "SpoIVA"
(→Extended information on the protein) |
(→References) |
||
| Line 149: | Line 149: | ||
== Reviews == | == Reviews == | ||
<pubmed>22192522 23202530</pubmed> | <pubmed>22192522 23202530</pubmed> | ||
| − | |||
== Original publications == | == Original publications == | ||
<pubmed>8748030, 15699190 1729246,8936302, 1729247,9922240,7592342, 8299942,1691789, 12644503, 18691972, 17427285, 19775244 22171814 15383836 23267091 19702880,22262582</pubmed> | <pubmed>8748030, 15699190 1729246,8936302, 1729247,9922240,7592342, 8299942,1691789, 12644503, 18691972, 17427285, 19775244 22171814 15383836 23267091 19702880,22262582</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:16, 4 January 2014
- Description: ATPase, spore coat morphogenetic protein, anchors the spore coat to the spore surface via SpoVM
| Gene name | spoIVA |
| Synonyms | spoVP |
| Essential | no |
| Product | ATPase, basement layer protein for spore coat assembly |
| Function | spore cortex formation and coat assembly |
| Gene expression levels in SubtiExpress: spoIVA | |
| Interactions involving this protein in SubtInteract: SpoIVA | |
| MW, pI | 55 kDa, 4.546 |
| Gene length, protein length | 1476 bp, 492 aa |
| Immediate neighbours | hbs, yphF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 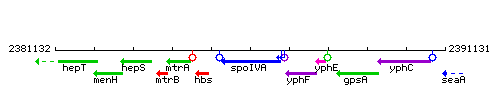
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed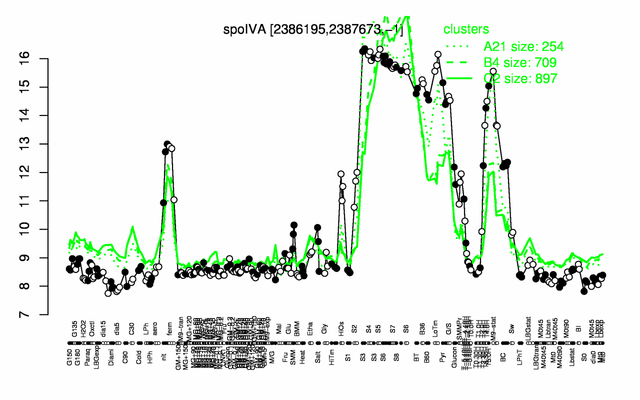
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
SpoIVA-dependent proteins of the spore coat basement
The gene
Basic information
- Locus tag: BSU22800
Phenotypes of a mutant
- the spore coat does not localize to the spore surface but self-assembles into aggregates in the mother cell cytoplasm PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- uses ATP hydrolysis to drive self-assembly into static filaments PubMed
- ATP hydrolysis drives polymerization of a nucleotide-free filament PubMed
- ploymerization depends on a critical threshold concentration of SpoIVA that is only achieved once the protein is recruited to the surface of the developing spore PubMed
- Protein family:
- belongs to the TRAFAC class of P-loop GTPases, but has lost the ability to bind GTP PubMed
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: contains a Walker A ATPase domain
- Modification:
- Effectors of protein activity:
- Localization:
- innermost protein of the spore coat basement PubMed
Database entries
- Structure:
- UniProt: P35149
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: spoIVA PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications