Difference between revisions of "OppA"
(→Biological materials) |
|||
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 94: | Line 90: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
** ([[OppD]]-[[OppF]])-([[OppB]]-[[OppC]])-[[OppA]] {{PubMed|10092453}} | ** ([[OppD]]-[[OppF]])-([[OppB]]-[[OppC]])-[[OppA]] {{PubMed|10092453}} | ||
| + | ** [[FloT]]-[[OppA]] {{PubMed|23651456}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| Line 117: | Line 114: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=oppA_1219849_1221486_1 oppA] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=oppA_1219849_1221486_1 oppA] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 150: | Line 147: | ||
=References= | =References= | ||
| − | + | <pubmed>1901616,10092453,12823818,11267663,9252573,10960106,,12823818,10383984,18957862 18763711 17218307, 7997159 20525796 23651456 21908671</pubmed> | |
| − | <pubmed>1901616,10092453,12823818,11267663,9252573,10960106,,12823818,10383984,18957862 18763711 17218307, 7997159 20525796</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:43, 10 May 2013
- Description: oligopeptide ABC transporter (binding protein)
| Gene name | oppA |
| Synonyms | spo0KA |
| Essential | no |
| Product | oligopeptide ABC transporter (binding protein) |
| Function | initiation of sporulation, competence development |
| Gene expression levels in SubtiExpress: oppA | |
| Interactions involving this protein in SubtInteract: OppA | |
| MW, pI | 61 kDa, 5.722 |
| Gene length, protein length | 1635 bp, 545 aa |
| Immediate neighbours | trpS, oppB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 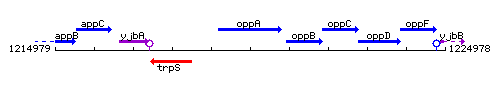
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
ABC transporters, utilization of nitrogen sources other than amino acids, genetic competence, phosphorelay, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU11430
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: bacterial solute-binding protein 5 family (according to Swiss-Prot)
- Paralogous protein(s): DppE
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on (Tyr-301 OR Tyr-303) AND Thr-470 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P24141
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
Biological materials
- Mutant: BP67 (spc) available in Fabian Commichau's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References