Difference between revisions of "MoaE"
| Line 1: | Line 1: | ||
| − | * '''Description:''' molybdopterin | + | * '''Description:''' molybdopterin synthase (large subunit), catalyses the transfer of sulfide from [[MoaD]] thiocarboxylate <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || molybdopterin | + | |style="background:#ABCDEF;" align="center"| '''Product''' || molybdopterin synthase (large subunit) |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || nitrate respiration | |style="background:#ABCDEF;" align="center"|'''Function''' || nitrate respiration | ||
| Line 60: | Line 60: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 68: | Line 66: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' catalyses the transfer of sulfide from [[MoaD]] thiocarboxylate |
* '''Protein family:''' moaE family (according to Swiss-Prot) | * '''Protein family:''' moaE family (according to Swiss-Prot) | ||
| Line 93: | Line 91: | ||
* '''Structure:''' | * '''Structure:''' | ||
| + | ** [http://www.rcsb.org/pdb/explore/explore.do?structureId=1NVI 1NVI] (the [[MoaD]]-[[MoaE]] complex from ''E. coli'') {{PubMed|12571227}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/O31705 O31705] | * '''UniProt:''' [http://www.uniprot.org/uniprot/O31705 O31705] | ||
| Line 104: | Line 103: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[mobA]]-[[moeB]]-[[moeA]]-[[mobB]]-[[moaE]]-[[moaD]]'' {{PubMed|22383849}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=moaE_1498966_1499439_1 moaE] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=moaE_1498966_1499439_1 moaE] {{PubMed|22383849}} | ||
| Line 135: | Line 134: | ||
=References= | =References= | ||
| − | + | == Reviews == | |
| + | <pubmed> 22616866 </pubmed> | ||
| + | == Original publications == | ||
| + | <pubmed> 12571227 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:34, 4 June 2012
- Description: molybdopterin synthase (large subunit), catalyses the transfer of sulfide from MoaD thiocarboxylate
| Gene name | moaE |
| Synonyms | |
| Essential | no |
| Product | molybdopterin synthase (large subunit) |
| Function | nitrate respiration |
| MW, pI | 17 kDa, 4.746 |
| Gene length, protein length | 471 bp, 157 aa |
| Immediate neighbours | mobB, moaD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 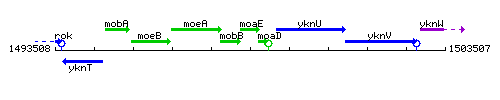
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed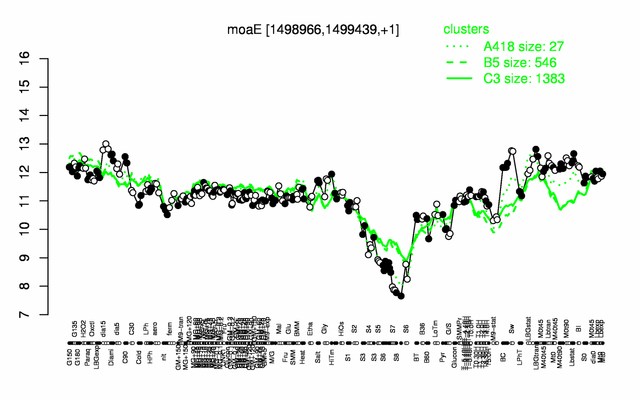
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14300
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: catalyses the transfer of sulfide from MoaD thiocarboxylate
- Protein family: moaE family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O31705
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Tadhg P Begley, Steven E Ealick, Fred W McLafferty
Thiamin biosynthesis: still yielding fascinating biological chemistry.
Biochem Soc Trans: 2012, 40(3);555-60
[PubMed:22616866]
[WorldCat.org]
[DOI]
(I p)
Original publications