Difference between revisions of "PyrH"
(→Database entries) |
|||
| Line 78: | Line 78: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2JJX 2JJX] (from ''Bacillus anthracis'', 42% identity, 64% similarity) {{PubMed|18625239}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/O31749 O31749] | * '''UniProt:''' [http://www.uniprot.org/uniprot/O31749 O31749] | ||
Revision as of 15:12, 19 February 2010
- Description: uridylate kinase
| Gene name | pyrH |
| Synonyms | smbA |
| Essential | no |
| Product | uridylate kinase |
| Function | pyrimidine biosynthesis |
| Metabolic function and regulation of this protein in SubtiPathways: Pyrimidines, Nucleotides (regulation) | |
| MW, pI | 25 kDa, 5.101 |
| Gene length, protein length | 720 bp, 240 aa |
| Immediate neighbours | tsf, frr |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 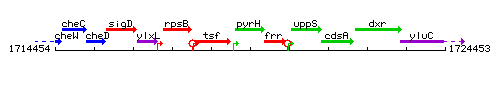
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU16510
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + UMP = ADP + UDP (according to Swiss-Prot)
- Protein family: UMP kinase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: O31749
- KEGG entry: [2]
- E.C. number: 2.7.4.22
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Cristina Gagyi, Nadia Bucurenci, Ovidiu Sîrbu, Gilles Labesse, Mihaela Ionescu, Augustin Ofiteru, Liliane Assairi, Stéphanie Landais, Antoine Danchin, Octavian Bârzu, Anne-Marie Gilles
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal activity.
Eur J Biochem: 2003, 270(15);3196-204
[PubMed:12869195]
[WorldCat.org]
[DOI]
(P p)
R J Turner, Y Lu, R L Switzer
Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism.
J Bacteriol: 1994, 176(12);3708-22
[PubMed:8206849]
[WorldCat.org]
[DOI]
(P p)
C L Quinn, B T Stephenson, R L Switzer
Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon.
J Biol Chem: 1991, 266(14);9113-27
[PubMed:1709162]
[WorldCat.org]
(P p)