Difference between revisions of "Pgm"
(→Expression and regulation) |
|||
| Line 95: | Line 95: | ||
* '''Operon:''' | * '''Operon:''' | ||
| − | ** ''[[cggR]]-[[gapA]]-[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' | + | ** ''[[cggR]]-[[gapA]]-[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' {{PubMed|11489127}} |
| − | ** ''[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' | + | ** ''[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' {{PubMed|11489127}} |
| − | * '''Sigma factor:''' [[SigA]] | + | * '''Sigma factor:''' [[SigA]] {{PubMed|11489127}} |
| − | * '''Regulation:''' expression activated by glucose ( | + | * '''Regulation:''' |
| − | ''[[cggR]]'': | + | ** expression activated by glucose (3.3 fold) [http://www.ncbi.nlm.nih.gov/pubmed/12850135 PubMed] |
| + | ** ''[[cggR]]'': induced by glycolytic substrates [[CggR]] {{PubMed|11489127}} | ||
| + | ** ''[[pgk]]'': constitutive {{PubMed|11489127}} | ||
| − | + | * '''Regulatory mechanism:''' transcription repression by [[CggR]] {{PubMed|11489127}} | |
| − | |||
| − | * '''Regulatory mechanism:''' transcription repression by [[CggR]] | ||
* '''Additional information:''' | * '''Additional information:''' | ||
Revision as of 19:52, 4 October 2009
- Description: phosphoglycerate mutase, glycolytic / gluconeogenic enzyme
| Gene name | pgm |
| Synonyms | gpmI |
| Essential | yes |
| Product | 2,3-bisphosphoglycerate-independent phosphoglycerate mutase |
| Function | enzyme in glycolysis / gluconeogenesis |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 56,1 kDa, 5.21 |
| Gene length, protein length | 1533 bp, 511 amino acids |
| Immediate neighbours | tpi, eno |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 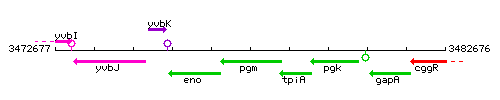
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU33910
Phenotypes of a mutant
- Essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2-phospho-D-glycerate = 3-phospho-D-glycerate (according to Swiss-Prot)
- Protein family: BPG-independent phosphoglycerate mutase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Reversible Michaelis-Menten PubMed
- Domains:
- Cofactor(s): Mn2+
- Effectors of protein activity:
- Interactions: Pgm-PfkA
- Localization: Cytoplasm (Homogeneous) PubMed
Database entries
- Structure: 1EJJ (Geobacillus stearothermophilus, complex with 3-phosphoglycerate), 1EQJ (Geobacillus stearothermophilus, complex with 2-phosphoglycerate), Geobacillus stearothermophilus, complex with 2-phosphoglycerate NCBI, Geobacillus stearothermophilus, complex with 3-phosphoglycerate NCBI
- UniProt: P39773
- KEGG entry: [3]
- E.C. number: 5.4.2.1]
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant: GP698 (cat), available in Stülke lab
- Expression vector:
- pGP1101 (N-terminal His-tag, in pWH844), available in Stülke lab
- pGP396 (Pgm-S62A, N-terminal His-tag, in pWH844), available in Stülke lab
- pGP92 (N-terminal Strep-tag, for SPINE, expression in B. subtilis, in pGP380), available in Stülke lab
- pGP1500 (expression in B. subtilis, in pBQ200), available in Stülke lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Mark J. Jedrzejas, Research Center Oakland, CA, USA Homepage
Your additional remarks
References