Difference between revisions of "PyrR"
| Line 80: | Line 80: | ||
* '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=1A3C 1A3C] (dimeric form) | * '''Structure:''' [http://www.rcsb.org/pdb/explore.do?structureId=1A3C 1A3C] (dimeric form) | ||
| − | * ''' | + | * '''UniProt:''' [http://www.uniprot.org/uniprot/P39765 P39765] |
* '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU15470] | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu:BSU15470] | ||
Revision as of 12:42, 20 July 2009
- Description: transcriptional antiterminator of the pyr operon
| Gene name | pyrR |
| Synonyms | |
| Essential | no |
| Product | transcriptional antiterminator with minor uracil phosphoribosyltransferase activity |
| Function | regulation of pyrimidine biosynthesis |
| Metabolic function and regulation of this protein in SubtiPathways: Pyrimidines, Nucleotides (regulation) | |
| MW, pI | 20 kDa, 4.991 |
| Gene length, protein length | 543 bp, 181 aa |
| Immediate neighbours | ylyB, pyrP |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 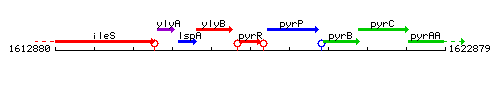
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU15470
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: UMP + diphosphate = uracil + 5-phospho-alpha-D-ribose 1-diphosphate (according to Swiss-Prot)
- Protein family: PyrR subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 1A3C (dimeric form)
- UniProt: P39765
- KEGG entry: [3]
- E.C. number: 2.4.2.9
Additional information
Expression and regulation
- Regulatory mechanism:
- PyrR: RNA switch, transcription termination/ antitermination (in the presence of uridine nucleotides: termination, in their absence: antitermination) PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References