Difference between revisions of "PnpA"
(→References) |
|||
| Line 122: | Line 122: | ||
=References= | =References= | ||
| − | + | ||
| − | + | <pubmed> 9811656, 19433509, 15995184, 8825778, 8636041, 15805522, 19193632, 1707536 </pubmed> | |
| − | + | ||
| − | |||
| − | |||
| − | |||
| − | |||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 12:58, 22 May 2009
- Description: polynucleotide phosphorylase
| Gene name | pnpA |
| Synonyms | comR |
| Essential | no |
| Product | polynucleotide phosphorylase (PNPase) (EC 2.7.7.8) |
| Function | necessary for competence development |
| MW, pI | 77 kDa, 4.89 |
| Gene length, protein length | 2115 bp, 705 aa |
| Immediate neighbours | rpsO, ylxY |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 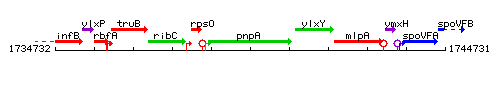
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 3'-5' exoribonuclease
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
Database entries
- Structure: 3CDI (protein from E. coli)
- Swiss prot entry:
- KEGG entry: BSU16690
- E.C. number:
Additional information
required for the expression of late competence genes comG and comK, requirement bypassed by a mecA disruption; may be necessary for modification of the srfA transcript (stabilization or translation activation)
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
David Bechhofer, Mount Sinai School, New York, USA Homepage
Your additional remarks
References
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed