Difference between revisions of "RpsR"
| Line 82: | Line 82: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| + | ** [[RpsF]]-[[RpsR]], the heterodimer binds the structure in the ''[[rpsF]]'' mRNA 5' UTR {{PubMed|24310371}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| Line 107: | Line 108: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' ''[[yyaF]]-[[rpsF]]-[[ssbA]]-[[rpsR]]'' {{PubMed|14762004}} | + | * '''Operon:''' |
| + | ** ''[[yyaF]]-[[rpsF]]-[[ssbA]]-[[rpsR]]'' {{PubMed|14762004}} | ||
| + | ** ''[[rpsF]]-[[ssbA]]-[[rpsR]]'' {{PubMed|24310371,22383849}} | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rpsR_4198603_4198842_-1 rpsR] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=rpsR_4198603_4198842_-1 rpsR] {{PubMed|22383849}} | ||
* '''[[Sigma factor]]:''' | * '''[[Sigma factor]]:''' | ||
| + | ** ''[[rpsF]]'': [[SigA]] {{PubMed|24310371}} | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 119: | Line 123: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | * '''Additional information:''' the mRNA is very stable (half-life > 15 min) [http://www.ncbi.nlm.nih.gov/sites/entrez/12884008 PubMed] | + | * '''Additional information:''' |
| + | ** the mRNA is very stable (half-life > 15 min) [http://www.ncbi.nlm.nih.gov/sites/entrez/12884008 PubMed] | ||
| + | ** the mRNA contains a structured motif in the 5' UTR upstream of the ''[[rpsF]]'' ORF that is bound by [[RpsF]]-[[RpsR]] heterodimers and may be implicated in autogenous regulation {{PubMed|24310371}} | ||
| + | |||
=Biological materials = | =Biological materials = | ||
| Line 140: | Line 147: | ||
=References= | =References= | ||
| − | <pubmed>12884008,14762004 ,11948146 19653700 11948165 23002217</pubmed> | + | <pubmed>12884008,14762004 ,11948146 19653700 11948165 23002217 22383849 24310371 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:30, 13 December 2013
- Description: ribosomal protein
| Gene name | rpsR |
| Synonyms | |
| Essential | yes PubMed |
| Product | ribosomal protein S18 |
| Function | translation |
| Gene expression levels in SubtiExpress: rpsR | |
| Interactions involving this protein in SubtInteract: RpsR | |
| MW, pI | 8 kDa, 11.494 |
| Gene length, protein length | 237 bp, 79 aa |
| Immediate neighbours | exoA, ssbA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 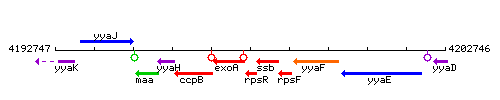
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
ComK regulon, stringent response
The gene
Basic information
- Locus tag: BSU40890
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein S18P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P21475
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References