Difference between revisions of "WprA"
(→References) |
|||
| Line 145: | Line 145: | ||
=References= | =References= | ||
| − | <pubmed> 22900538 22923395 23180473 | + | <pubmed> 22900538 22923395 23180473 21815947 10075409,11987133,9004506,20525796,18957862 18763711 16306698, 9687444 12028417 </pubmed> |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:18, 13 July 2013
- Description: secreted quality control protease
| Gene name | wprA |
| Synonyms | yisM |
| Essential | no |
| Product | secreted quality control protease |
| Function | protein quality control |
| Gene expression levels in SubtiExpress: wprA | |
| Interactions involving this protein in SubtInteract: WprA | |
| MW, pI | 96 kDa, 9.58 |
| Gene length, protein length | 2682 bp, 894 aa |
| Immediate neighbours | yisL, yisN |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 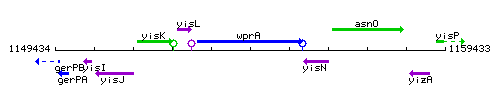
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed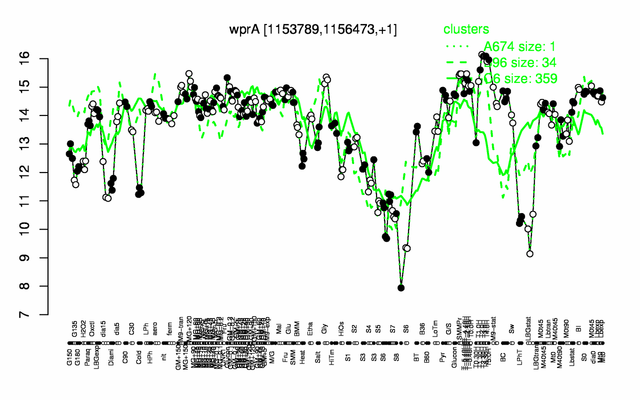
| |
Contents
Categories containing this gene/protein
cell wall/ other, proteolysis,
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU10770
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase S8 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P54423
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
- An antisense RNA is predicted forwprA PubMed
- the amount of the mRNA is substantially decreased upon depletion of RNase Y PubMed
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References