Difference between revisions of "DctP"
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 82: | Line 78: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 97: | Line 93: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://pdb.org/pdb/explore/explore.do?structureId=4KY0 4KY0] (the glutamate transporter of ''Thermococcus kodakarensis'', 31% identity, 71% similarity) {{PubMed|24013209}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P96603 P96603] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P96603 P96603] | ||
| Line 115: | Line 111: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dctP_500166_501431_1 dctP] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dctP_500166_501431_1 dctP] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
** ''[[dctP]]'': [[SigA]] {{PubMed|10708364}} | ** ''[[dctP]]'': [[SigA]] {{PubMed|10708364}} | ||
| Line 149: | Line 145: | ||
=References= | =References= | ||
| − | <pubmed>12850135, 10708364 10627041, 12949159 20363944 22389480 22900538 </pubmed> | + | <pubmed>12850135, 10708364 10627041, 12949159 20363944 22389480 22900538 24013209</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:04, 27 December 2013
- Description: uptake of succinate, fumurate, malate and oxaloacetate via proton symport
| Gene name | dctP |
| Synonyms | ydbH, dctA |
| Essential | no |
| Product | C4-dicarboxylate transport protein |
| Function | uptake of succinate, fumurate, malate and oxaloacetate |
| Gene expression levels in SubtiExpress: dctP | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 45 kDa, 8.668 |
| Gene length, protein length | 1263 bp, 421 aa |
| Immediate neighbours | dctR, ydbI |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 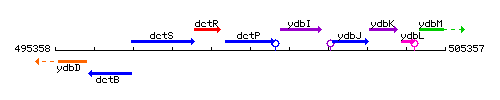
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed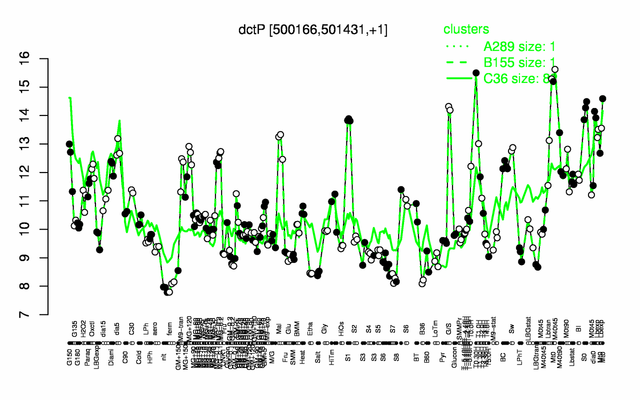
| |
Contents
Categories containing this gene/protein
transporters/ other, utilization of specific carbon sources, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU04470
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: uptake of succinate, fumurate, malate and oxaloacetate via proton symport PubMed
- Protein family: View classification (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization:
- cell membrane (according to Swiss-Prot)
Database entries
- Structure: 4KY0 (the glutamate transporter of Thermococcus kodakarensis, 31% identity, 71% similarity) PubMed
- UniProt: P96603
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Sonja Jensen, Albert Guskov, Stephan Rempel, Inga Hänelt, Dirk Jan Slotboom
Crystal structure of a substrate-free aspartate transporter.
Nat Struct Mol Biol: 2013, 20(10);1224-6
[PubMed:24013209]
[WorldCat.org]
[DOI]
(I p)
Bogumiła C Marciniak, Monika Pabijaniak, Anne de Jong, Robert Dűhring, Gerald Seidel, Wolfgang Hillen, Oscar P Kuipers
High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis.
BMC Genomics: 2012, 13;401
[PubMed:22900538]
[WorldCat.org]
[DOI]
(I e)
Gregory T Smaldone, Olga Revelles, Ahmed Gaballa, Uwe Sauer, Haike Antelmann, John D Helmann
A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism.
J Bacteriol: 2012, 194(10);2594-605
[PubMed:22389480]
[WorldCat.org]
[DOI]
(I p)
Maarten Groeneveld, Ruud G J Detert Oude Weme, Ria H Duurkens, Dirk Jan Slotboom
Biochemical characterization of the C4-dicarboxylate transporter DctA from Bacillus subtilis.
J Bacteriol: 2010, 192(11);2900-7
[PubMed:20363944]
[WorldCat.org]
[DOI]
(I p)
Kousei Tanaka, Kazuo Kobayashi, Naotake Ogasawara
The Bacillus subtilis YufLM two-component system regulates the expression of the malate transporters MaeN (YufR) and YflS, and is essential for utilization of malate in minimal medium.
Microbiology (Reading): 2003, 149(Pt 9);2317-2329
[PubMed:12949159]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
Kei Asai, Sang-Hoon Baik, Yasuhiro Kasahara, Shigeki Moriya, Naotake Ogasawara
Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis.
Microbiology (Reading): 2000, 146 ( Pt 2);263-271
[PubMed:10708364]
[WorldCat.org]
[DOI]
(P p)
Ralf Rabus, Donald L Jack, David J Kelly, Milton H Saier
TRAP transporters: an ancient family of extracytoplasmic solute-receptor-dependent secondary active transporters.
Microbiology (Reading): 1999, 145 ( Pt 12);3431-3445
[PubMed:10627041]
[WorldCat.org]
[DOI]
(P p)