Difference between revisions of "CypC"
Raphael2215 (talk | contribs) (→Database entries) |
|||
| Line 93: | Line 93: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2ZQJ 2ZQJ] {{PubMed|12519760}} | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2ZQJ 2ZQJ] [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1IZO 1IZO]{{PubMed|12519760}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/O31440 O31440] | * '''UniProt:''' [http://www.uniprot.org/uniprot/O31440 O31440] | ||
Revision as of 08:46, 10 July 2013
- Description: long chain-fatty acid beta-hydroxylating cytochrome P450, H(2)O(2)-dependent, hydroxylates myristic acid to beta-hydroxymyristic acid
| Gene name | cypC |
| Synonyms | ybdT |
| Essential | no |
| Product | fatty acid beta-hydroxylating cytochrome P450 |
| Function | biosynthesis of beta-hydroxy fatty acid for lipopeptides |
| Gene expression levels in SubtiExpress: cypC | |
| MW, pI | 47 kDa, 6.468 |
| Gene length, protein length | 1251 bp, 417 aa |
| Immediate neighbours | ybxI, ybyB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 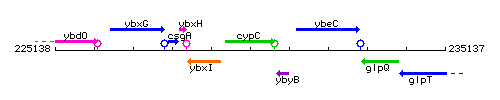
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed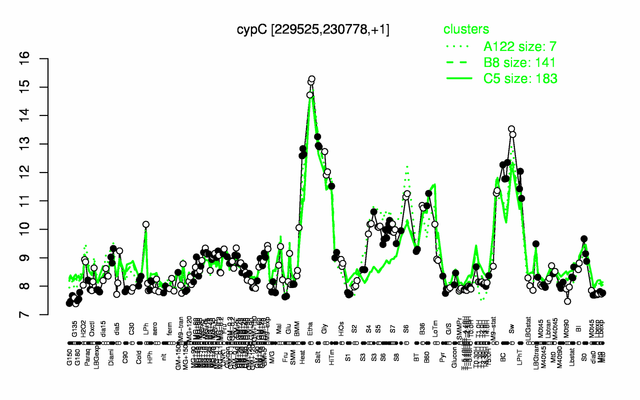
| |
Contents
Categories containing this gene/protein
general stress proteins (controlled by SigB), electron transport/ other, lipid metabolism/ other
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU02100
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- hydroxylation of a long-chain fatty acid (e.g. myristic acid) at the alpha- and beta-positions using hydrogen peroxide as an oxidant PubMed
- Protein family: cytochrome P450 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O31440
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: cypC (according to DBTBS)
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Marco Girhard, Elmar Kunigk, Svetlana Tihovsky, Victoria V Shumyantseva, Vlada B Urlacher
Light-driven biocatalysis with cytochrome P450 peroxygenases.
Biotechnol Appl Biochem: 2013, 60(1);111-8
[PubMed:23586998]
[WorldCat.org]
[DOI]
(I p)
Noha H Youssef, Neil Wofford, Michael J McInerney
Importance of the long-chain fatty acid beta-hydroxylating cytochrome P450 enzyme YbdT for lipopeptide biosynthesis in Bacillus subtilis strain OKB105.
Int J Mol Sci: 2011, 12(3);1767-86
[PubMed:21673922]
[WorldCat.org]
[DOI]
(I p)
Osami Shoji, Takashi Fujishiro, Shingo Nagano, Shota Tanaka, Takuya Hirose, Yoshitsugu Shiro, Yoshihito Watanabe
Understanding substrate misrecognition of hydrogen peroxide dependent cytochrome P450 from Bacillus subtilis.
J Biol Inorg Chem: 2010, 15(8);1331-9
[PubMed:20697922]
[WorldCat.org]
[DOI]
(I p)
Osami Shoji, Takashi Fujishiro, Hiroshi Nakajima, Misa Kim, Shingo Nagano, Yoshitsugu Shiro, Yoshihito Watanabe
Hydrogen peroxide dependent monooxygenations by tricking the substrate recognition of cytochrome P450BSbeta.
Angew Chem Int Ed Engl: 2007, 46(20);3656-9
[PubMed:17385817]
[WorldCat.org]
[DOI]
(P p)
Dirk Höper, Uwe Völker, Michael Hecker
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.
J Bacteriol: 2005, 187(8);2810-26
[PubMed:15805528]
[WorldCat.org]
[DOI]
(P p)
Dong-Sun Lee, Akari Yamada, Hiroshi Sugimoto, Isamu Matsunaga, Hisashi Ogura, Kosuke Ichihara, Shin-Ichi Adachi, Sam-Yong Park, Yoshitsugu Shiro
Substrate recognition and molecular mechanism of fatty acid hydroxylation by cytochrome P450 from Bacillus subtilis. Crystallographic, spectroscopic, and mutational studies.
J Biol Chem: 2003, 278(11);9761-7
[PubMed:12519760]
[WorldCat.org]
[DOI]
(P p)
Isamu Matsunaga, Tatsuo Sumimoto, Minoru Ayata, Hisashi Ogura
Functional modulation of a peroxygenase cytochrome P450: novel insight into the mechanisms of peroxygenase and peroxidase enzymes.
FEBS Lett: 2002, 528(1-3);90-4
[PubMed:12297285]
[WorldCat.org]
[DOI]
(P p)
Dong Sun Lee, Akari Yamada, Isamu Matsunaga, Kosuke Ichihara, Shin-ichi Adachi, Sam-Yong Park, Yoshitsugu Shiro
Crystallization and preliminary X-ray diffraction analysis of fatty-acid hydroxylase cytochrome P450BSbeta from Bacillus subtilis.
Acta Crystallogr D Biol Crystallogr: 2002, 58(Pt 4);687-9
[PubMed:11914497]
[WorldCat.org]
[DOI]
(P p)
Isamu Matsunaga, Akari Yamada, Dong-Sun Lee, Eiji Obayashi, Nagatoshi Fujiwara, Kazuo Kobayashi, Hisashi Ogura, Yoshitsugu Shiro
Enzymatic reaction of hydrogen peroxide-dependent peroxygenase cytochrome P450s: kinetic deuterium isotope effects and analyses by resonance Raman spectroscopy.
Biochemistry: 2002, 41(6);1886-92
[PubMed:11827534]
[WorldCat.org]
[DOI]
(P p)
I Matsunaga, A Ueda, T Sumimoto, K Ichihara, M Ayata, H Ogura
Site-directed mutagenesis of the putative distal helix of peroxygenase cytochrome P450.
Arch Biochem Biophys: 2001, 394(1);45-53
[PubMed:11566026]
[WorldCat.org]
[DOI]
(P p)
I Matsunaga, A Ueda, N Fujiwara, T Sumimoto, K Ichihara
Characterization of the ybdT gene product of Bacillus subtilis: novel fatty acid beta-hydroxylating cytochrome P450.
Lipids: 1999, 34(8);841-6
[PubMed:10529095]
[WorldCat.org]
[DOI]
(P p)