Difference between revisions of "MurR"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || probably regulation of muramic acid utilization | |style="background:#ABCDEF;" align="center"|'''Function''' || probably regulation of muramic acid utilization | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU01690 murR] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/murein/index.html Murein recycling]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/murein/index.html Murein recycling]''' | ||
| Line 24: | Line 24: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[murP]]'', ''[[murQ]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[murP]]'', ''[[murQ]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU01690 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU01690 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU01690 Advanced_DNA] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:ybbH_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:ybbH_context.gif]] | ||
Revision as of 12:00, 13 May 2013
- Description: probably transcriptional regulator of genes required for the utilization of N-acetylmuramic acid, homolog to E. coli MurR
| Gene name | murR |
| Synonyms | ybbH |
| Essential | no |
| Product | transcriptional repressor |
| Function | probably regulation of muramic acid utilization |
| Gene expression levels in SubtiExpress: murR | |
| Metabolic function and regulation of this protein in SubtiPathways: Murein recycling | |
| MW, pI | 30 kDa, 6.873 |
| Gene length, protein length | 849 bp, 283 aa |
| Immediate neighbours | murP, murQ |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 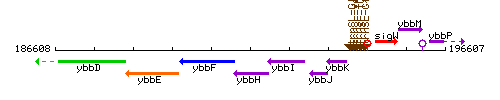
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed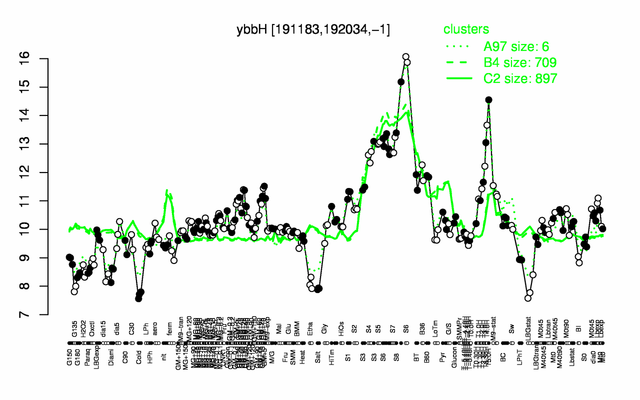
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover, utilization of specific carbon sources, transcription factors and their control
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01690
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 2O3F (N-terminal domain)
- UniProt: Q45581
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References