Difference between revisions of "Sfp/2"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 1: | Line 1: | ||
| − | * '''Description:''' 4'- | + | * '''Description:''' 4'-phosphopantetheinyl transferase (surfactin synthetase-activating enzyme), inactive pseudogene in strain 168<br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 10: | Line 10: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || 4'- | + | |style="background:#ABCDEF;" align="center"| '''Product''' || 4'-phosphopantetheinyl transferase |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || phosphopantetheinylates a serine residue in each<br/> of the seven peptidyl carrier protein domains of the <br/>first three subunits of surfactin synthetase ([[SrfAA]]-[[SrfAB]]-[[SrfAC]]) and [[AcpK]] | |style="background:#ABCDEF;" align="center"|'''Function''' || phosphopantetheinylates a serine residue in each<br/> of the seven peptidyl carrier protein domains of the <br/>first three subunits of surfactin synthetase ([[SrfAA]]-[[SrfAB]]-[[SrfAC]]) and [[AcpK]] | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || | + | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || - , - |
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 495 bp, | + | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 495 bp, - |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[sfp/1]]'', ''[[yczE]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[sfp/1]]'', ''[[yczE]]'' | ||
Revision as of 17:21, 9 February 2012
- Description: 4'-phosphopantetheinyl transferase (surfactin synthetase-activating enzyme), inactive pseudogene in strain 168
| Gene name | sfp/1 |
| Synonyms | sfp |
| Essential | no |
| Product | 4'-phosphopantetheinyl transferase |
| Function | phosphopantetheinylates a serine residue in each of the seven peptidyl carrier protein domains of the first three subunits of surfactin synthetase (SrfAA-SrfAB-SrfAC) and AcpK |
| MW, pI | - , - |
| Gene length, protein length | 495 bp, - |
| Immediate neighbours | sfp/1, yczE |
| Gene sequence (+200bp) | Protein sequence |
| Caution: The sequence for this gene in SubtiList contains errors | |
Genetic context 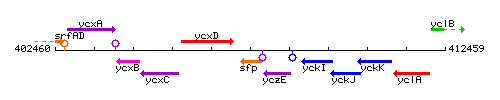
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biofilm formation, biosynthesis of antibacterial compounds, pseudogenes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU03570
Phenotypes of a mutant
No swarming motility on B medium. PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
- This gene is a pseudogene in B. subtilis 168 due to a mutation that resulted in an internal frameshift. The second part of the pseudogene is sfp/2.
- Correction of sfp, epsC, swrAA, and degQ as well as introduction of rapP from a plasmid present in NCIB3610 results in biofilm formation in B. subtilis 168 PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: CoA + apo-[peptidyl-carrier protein] = adenosine 3',5'-bisphosphate + holo-[peptidyl-carrier protein] (according to Swiss-Prot)
- Protein family: Gsp/sfp/hetI/acpT family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1QR0 (complex with CoA)
- Swiss prot entry:
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Mohamed Marahiel, Marburg University, Germany homepage
Your additional remarks
References
Reviews
Original publications