Difference between revisions of "AcoC"
| Line 123: | Line 123: | ||
| − | <pubmed>16479537,11274109 </pubmed> | + | <pubmed>11274109,10368162,,16479537,11274109 </pubmed> |
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 21:12, 13 June 2009
- Description: acetoin dehydrogenase E2 component (dihydrolipoamide acetyltransferase)
| Gene name | acoC |
| Synonyms | yfjI |
| Essential | no |
| Product | acetoin dehydrogenase E2 component (dihydrolipoamide acetyltransferase) |
| Function | acetoin utilization |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 42 kDa, 6.524 |
| Gene length, protein length | 1194 bp, 398 aa |
| Immediate neighbours | acoB, acoL |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 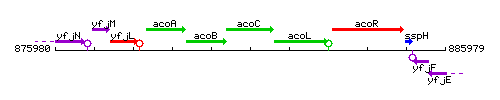
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU08080
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Acetyl-CoA + enzyme N(6)-(dihydrolipoyl)lysine = CoA + enzyme N(6)-(S-acetyldihydrolipoyl)lysine (according to Swiss-Prot)
- Protein family: lipoyl-binding domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: Membrane-proximal (Spotty) PubMed
Database entries
- Structure:
- Swiss prot entry: O31550
- KEGG entry: [3]
- E.C. number: 2.3.1.12
Additional information
Expression and regulation
- Regulatory mechanism: CcpA: transcription repression, AcoR: transcription activation (interaction with SigL-containing RNA polymerase) PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Michel Debarbouille, Pasteur Institute, Paris, France Homepage
Your additional remarks
References
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed