Difference between revisions of "RpoC"
(→Original publications) |
|||
| Line 100: | Line 100: | ||
** [[SigF]]-([[RpoB]]-[[RpoC]]), [[SigG]]-([[RpoB]]-[[RpoC]]) | ** [[SigF]]-([[RpoB]]-[[RpoC]]), [[SigG]]-([[RpoB]]-[[RpoC]]) | ||
** [[SigH]]-([[RpoB]]-[[RpoC]]), [[SigI]]-([[RpoB]]-[[RpoC]]) | ** [[SigH]]-([[RpoB]]-[[RpoC]]), [[SigI]]-([[RpoB]]-[[RpoC]]) | ||
| − | ** [[SigK]]-([[RpoB]]-[[RpoC]]) | + | ** [[SigK]]-([[RpoB]]-[[RpoC]]) |
| + | ** [[SigL]]-([[RpoB]]-[[RpoC]]-[[RpoA]]) {{PubMed|26293966}} | ||
** [[SigM]]-([[RpoB]]-[[RpoC]]), [[SigV]]-([[RpoB]]-[[RpoC]]) | ** [[SigM]]-([[RpoB]]-[[RpoC]]), [[SigV]]-([[RpoB]]-[[RpoC]]) | ||
** [[SigW]]-([[RpoB]]-[[RpoC]]), [[SigX]]-([[RpoB]]-[[RpoC]]) | ** [[SigW]]-([[RpoB]]-[[RpoC]]), [[SigX]]-([[RpoB]]-[[RpoC]]) | ||
| Line 164: | Line 165: | ||
<pubmed> 22210308 24763425 </pubmed> | <pubmed> 22210308 24763425 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>7657605,12486038,10675340,7592585, 19735077,18763711, 19680289 22517742 23070162,20817769 24572018 25092033 24491578 26243774</pubmed> | + | <pubmed>7657605,12486038,10675340,7592585, 19735077,18763711, 19680289 22517742 23070162,20817769 24572018 25092033 24491578 26243774 26293966</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:28, 30 August 2015
- Description: RNA polymerase beta' subunit
| Gene name | rpoC |
| Synonyms | |
| Essential | yes PubMed |
| Product | RNA polymerase beta' subunit |
| Function | transcription |
| Gene expression levels in SubtiExpress: rpoC | |
| Interactions involving this protein in SubtInteract: RpoC | |
| MW, pI | 133 kDa, 8.863 |
| Gene length, protein length | 3597 bp, 1199 aa |
| Immediate neighbours | rpoB, ybxF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed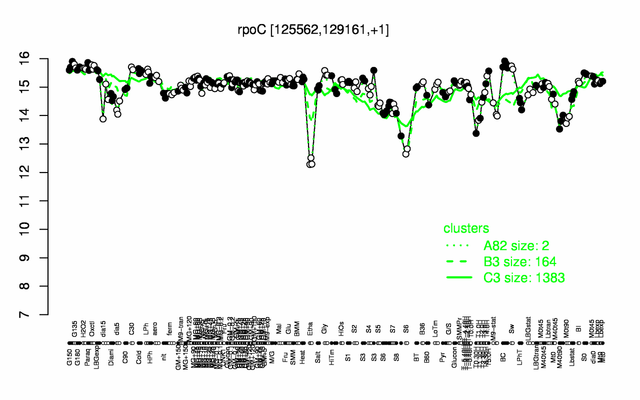
| |
Contents
Categories containing this gene/protein
transcription, essential genes, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01080
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU01080
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
- mutations in mtrB, sigB, rpoB, and rpoC allow B. subtilis to grow with 4-fluorotryptophan rather than with tryptophan as a canonical amino acid of the genetic code PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1) (according to Swiss-Prot)
- Protein family: RNA polymerase beta' chain family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- phosphorylated on Arg-335 and Arg-803 PubMed
- Effectors of protein activity:
- Interactions:
- RpoA-RpoB-RpoC PubMed
- RpoC-RpoY PubMed
- SigA-(RpoB-RpoC) PubMed, SigB-(RpoB-RpoC)
- SigD-(RpoB-RpoC), SigE-(RpoB-RpoC)
- SigF-(RpoB-RpoC), SigG-(RpoB-RpoC)
- SigH-(RpoB-RpoC), SigI-(RpoB-RpoC)
- SigK-(RpoB-RpoC)
- SigL-(RpoB-RpoC-RpoA) PubMed
- SigM-(RpoB-RpoC), SigV-(RpoB-RpoC)
- SigW-(RpoB-RpoC), SigX-(RpoB-RpoC)
- SigY-(RpoB-RpoC), SigZ-(RpoB-RpoC)
- Xpf-(RpoB-RpoC), YlaC-(RpoB-RpoC)
- YvrHa-RpoC PubMed
- RpoC-RpoE (demonstrated for S. aureus) PubMed
Database entries
- BsubCyc: BSU01080
- Structure:
- UniProt: P37871
- KEGG entry: [2]
- E.C. number: 2.7.7.6
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Fabian Commichau's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Nikolay Zenkin
Multiple personalities of the RNA polymerase active centre.
Microbiology (Reading): 2014, 160(Pt 7);1316-1320
[PubMed:24763425]
[WorldCat.org]
[DOI]
(I p)
Lakshminarayan M Iyer, L Aravind
Insights from the architecture of the bacterial transcription apparatus.
J Struct Biol: 2012, 179(3);299-319
[PubMed:22210308]
[WorldCat.org]
[DOI]
(I p)
Original publications
Yun Yang, Vidya C Darbari, Nan Zhang, Duo Lu, Robert Glyde, Yi-Ping Wang, Jared T Winkelman, Richard L Gourse, Katsuhiko S Murakami, Martin Buck, Xiaodong Zhang
TRANSCRIPTION. Structures of the RNA polymerase-σ54 reveal new and conserved regulatory strategies.
Science: 2015, 349(6250);882-5
[PubMed:26293966]
[WorldCat.org]
[DOI]
(I p)
Andrea Volante, Begoña Carrasco, Mariangela Tabone, Juan C Alonso
The interaction of ω2 with the RNA polymerase β' subunit functions as an activation to repression switch.
Nucleic Acids Res: 2015, 43(19);9249-61
[PubMed:26243774]
[WorldCat.org]
[DOI]
(I p)
Andrew N Keller, Xiao Yang, Jana Wiedermannová, Olivier Delumeau, Libor Krásný, Peter J Lewis
ε, a new subunit of RNA polymerase found in gram-positive bacteria.
J Bacteriol: 2014, 196(20);3622-32
[PubMed:25092033]
[WorldCat.org]
[DOI]
(I p)
Allen Chi-Shing Yu, Aldrin Kay-Yuen Yim, Wai-Kin Mat, Amy Hin-Yan Tong, Si Lok, Hong Xue, Stephen Kwok-Wing Tsui, J Tze-Fei Wong, Ting-Fung Chan
Mutations enabling displacement of tryptophan by 4-fluorotryptophan as a canonical amino acid of the genetic code.
Genome Biol Evol: 2014, 6(3);629-41
[PubMed:24572018]
[WorldCat.org]
[DOI]
(I p)
Andy Weiss, J Antonio Ibarra, Jessica Paoletti, Ronan K Carroll, Lindsey N Shaw
The δ subunit of RNA polymerase guides promoter selectivity and virulence in Staphylococcus aureus.
Infect Immun: 2014, 82(4);1424-35
[PubMed:24491578]
[WorldCat.org]
[DOI]
(I p)
Yong Heon Lee, Ki Hyun Nam, John D Helmann
A mutation of the RNA polymerase β' subunit (rpoC) confers cephalosporin resistance in Bacillus subtilis.
Antimicrob Agents Chemother: 2013, 57(1);56-65
[PubMed:23070162]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Shu Ishikawa, Taku Oshima, Ken Kurokawa, Yoko Kusuya, Naotake Ogasawara
RNA polymerase trafficking in Bacillus subtilis cells.
J Bacteriol: 2010, 192(21);5778-87
[PubMed:20817769]
[WorldCat.org]
[DOI]
(I p)
Elecia B Johnston, Peter J Lewis, Renate Griffith
The interaction of Bacillus subtilis sigmaA with RNA polymerase.
Protein Sci: 2009, 18(11);2287-97
[PubMed:19735077]
[WorldCat.org]
[DOI]
(I p)
Xiao Yang, Seeseei Molimau, Geoff P Doherty, Elecia B Johnston, Jon Marles-Wright, Rosalba Rothnagel, Ben Hankamer, Richard J Lewis, Peter J Lewis
The structure of bacterial RNA polymerase in complex with the essential transcription elongation factor NusA.
EMBO Rep: 2009, 10(9);997-1002
[PubMed:19680289]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Claudia Rollenhagen, Haike Antelmann, Janine Kirstein, Olivier Delumeau, Michael Hecker, Michael D Yudkin
Binding of sigma(A) and sigma(B) to core RNA polymerase after environmental stress in Bacillus subtilis.
J Bacteriol: 2003, 185(1);35-40
[PubMed:12486038]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, S D Thaker, J Errington
Compartmentalization of transcription and translation in Bacillus subtilis.
EMBO J: 2000, 19(4);710-8
[PubMed:10675340]
[WorldCat.org]
[DOI]
(P p)
X Yang, C W Price
Streptolydigin resistance can be conferred by alterations to either the beta or beta' subunits of Bacillus subtilis RNA polymerase.
J Biol Chem: 1995, 270(41);23930-3
[PubMed:7592585]
[WorldCat.org]
[DOI]
(P p)
K J Boor, M L Duncan, C W Price
Genetic and transcriptional organization of the region encoding the beta subunit of Bacillus subtilis RNA polymerase.
J Biol Chem: 1995, 270(35);20329-36
[PubMed:7657605]
[WorldCat.org]
[DOI]
(P p)