Difference between revisions of "SigM"
(→References) |
(→Phenotypes of a mutant) |
||
| Line 57: | Line 57: | ||
* ''sigM'' mutants form aberrantly shaped cells, which swell and lyse spontaneously during growth in NB medium containing increased levels (0.35-0.7 M) of a wide range of different salts {{PubMed|10216858}} | * ''sigM'' mutants form aberrantly shaped cells, which swell and lyse spontaneously during growth in NB medium containing increased levels (0.35-0.7 M) of a wide range of different salts {{PubMed|10216858}} | ||
* increased sensitivity towards beta-lactam antibiotics {{PubMed|22211522}} | * increased sensitivity towards beta-lactam antibiotics {{PubMed|22211522}} | ||
| + | * a ''[[sigM]] [[murJ]]'' double mutant is not viable (due to the lack of both lipid II flippases) {{PubMed|25918422}} | ||
=== Database entries === | === Database entries === | ||
Revision as of 13:00, 8 May 2015
- Description: RNA polymerase ECF-type sigma factor SigM
| Gene name | sigM |
| Synonyms | yhdM |
| Essential | no |
| Product | RNA polymerase ECF-type sigma factor SigM |
| Function | responsible for intrinsic resistance against beta-lactam antibiotics |
| Gene expression levels in SubtiExpress: sigM | |
| Interactions involving this protein in SubtInteract: SigM | |
| MW, pI | 19 kDa, 6.77 |
| Gene length, protein length | 489 bp, 163 aa |
| Immediate neighbours | yhdL, yhdN |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 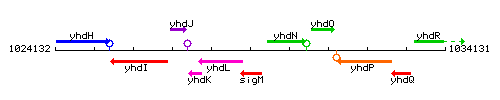
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription, sigma factors and their control, cell envelope stress proteins (controlled by SigM, V, W, X, Y), resistance against toxins/ antibiotics
This gene is a member of the following regulons
The SigM regulon
The gene
Basic information
- Locus tag: BSU09520
Phenotypes of a mutant
- SigM is essential for growth and survival in nutrient broth (NB) containing 1.4 M NaCl PubMed
- sigM mutants form aberrantly shaped cells, which swell and lyse spontaneously during growth in NB medium containing increased levels (0.35-0.7 M) of a wide range of different salts PubMed
- increased sensitivity towards beta-lactam antibiotics PubMed
- a sigM murJ double mutant is not viable (due to the lack of both lipid II flippases) PubMed
Database entries
- BsubCyc: BSU09520
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: sigma-70 factor family, ECF subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU09520
- Structure:
- UniProt: O07582
- KEGG entry: [3]
- E.C. number:
Additional information
- Expression of the SigM regulon in increased in ugtP mutants PubMed
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- 1A906 (sigM::kan), PubMed, available at BGSC
- BP129 (sigM::aphA3), available in Fabian Commichau's lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Reviews
Bianca Mendes Souza, Thiago Luiz de Paula Castro, Rodrigo Dias de Oliveira Carvalho, Nubia Seyffert, Artur Silva, Anderson Miyoshi, Vasco Azevedo
σ(ECF) factors of gram-positive bacteria: a focus on Bacillus subtilis and the CMNR group.
Virulence: 2014, 5(5);587-600
[PubMed:24921931]
[WorldCat.org]
[DOI]
(I p)
The SigM regulon
Other original publications