Difference between revisions of "YisP"
| Line 1: | Line 1: | ||
| − | * '''Description:''' production of | + | * '''Description:''' farnesyl diphosphate phosphatase, production of farnesol <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
| Line 9: | Line 9: | ||
|style="background:#ABCDEF;" align="center"| '''Essential''' || no | |style="background:#ABCDEF;" align="center"| '''Essential''' || no | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"| '''Product''' || | + | |style="background:#ABCDEF;" align="center"| '''Product''' || farnesyl diphosphate phosphatase |
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || control of [[KinC]] activity | |style="background:#ABCDEF;" align="center"|'''Function''' || control of [[KinC]] activity | ||
| Line 53: | Line 53: | ||
* complete loss of pellicle-forming ability {{PubMed|20713508}} | * complete loss of pellicle-forming ability {{PubMed|20713508}} | ||
* reduced [[protein secretion]] {{PubMed|23651456}} | * reduced [[protein secretion]] {{PubMed|23651456}} | ||
| + | |||
=== Database entries === | === Database entries === | ||
* '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU10810&redirect=T BSU10810] | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU10810&redirect=T BSU10810] | ||
| Line 61: | Line 62: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 70: | Line 69: | ||
* '''Catalyzed reaction/ biological activity:''' | * '''Catalyzed reaction/ biological activity:''' | ||
| + | ** farnesyl diphosphate --> farnesol {{PubMed|25308276}} | ||
* '''Protein family:''' | * '''Protein family:''' | ||
| Line 94: | Line 94: | ||
* '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU10810&redirect=T BSU10810] | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU10810&redirect=T BSU10810] | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://pdb.org/pdb/explore/explore.do?structureId=3WE9 3WE9] {{PubMed|25308276}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/O06728 O06728] | * '''UniProt:''' [http://www.uniprot.org/uniprot/O06728 O06728] | ||
| Line 103: | Line 103: | ||
=== Additional information=== | === Additional information=== | ||
| − | + | ||
=Expression and regulation= | =Expression and regulation= | ||
| Line 140: | Line 140: | ||
=References= | =References= | ||
| − | <pubmed> 20713508 19935659 23651456 23295493</pubmed> | + | <pubmed> 20713508 19935659 23651456 23295493 25308276 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:05, 14 October 2014
- Description: farnesyl diphosphate phosphatase, production of farnesol
| Gene name | yisP |
| Synonyms | yucD |
| Essential | no |
| Product | farnesyl diphosphate phosphatase |
| Function | control of KinC activity |
| Gene expression levels in SubtiExpress: yisP | |
| MW, pI | 31 kDa, 7.258 |
| Gene length, protein length | 822 bp, 274 aa |
| Immediate neighbours | yizA, yisQ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 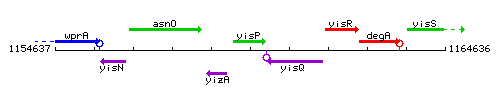
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, phosphorelay, biofilm formation
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU10810
Phenotypes of a mutant
- complete loss of pellicle-forming ability PubMed
- reduced protein secretion PubMed
Database entries
- BsubCyc: BSU10810
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- farnesyl diphosphate --> farnesol PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): NADH PubMed
- Effectors of protein activity:
Database entries
- BsubCyc: BSU10810
- UniProt: O06728
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 37 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Xinxin Feng, Yumei Hu, Yingying Zheng, Wei Zhu, Kai Li, Chun-Hsiang Huang, Tzu-Ping Ko, Feifei Ren, Hsiu-Chien Chan, Mulugeta Nega, Shannon Bogue, Daniel López, Roberto Kolter, Friedrich Götz, Rey-Ting Guo, Eric Oldfield
Structural and functional analysis of Bacillus subtilis YisP reveals a role of its product in biofilm production.
Chem Biol: 2014, 21(11);1557-63
[PubMed:25308276]
[WorldCat.org]
[DOI]
(I p)
Juri Niño Bach, Marc Bramkamp
Flotillins functionally organize the bacterial membrane.
Mol Microbiol: 2013, 88(6);1205-17
[PubMed:23651456]
[WorldCat.org]
[DOI]
(I p)
Yumei Hu, Shiru Jia, Feifei Ren, Chun Hsiang Huang, Tzu Ping Ko, Douglas A Mitchell, Rey Ting Guo, Yingying Zheng
Crystallization and preliminary X-ray diffraction analysis of YisP protein from Bacillus subtilis subsp. subtilis strain 168.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2013, 69(Pt 1);77-9
[PubMed:23295493]
[WorldCat.org]
[DOI]
(I p)
Daniel López, Roberto Kolter
Functional microdomains in bacterial membranes.
Genes Dev: 2010, 24(17);1893-902
[PubMed:20713508]
[WorldCat.org]
[DOI]
(I p)
Tzu-Lin Hsiao, Olga Revelles, Lifeng Chen, Uwe Sauer, Dennis Vitkup
Automatic policing of biochemical annotations using genomic correlations.
Nat Chem Biol: 2010, 6(1);34-40
[PubMed:19935659]
[WorldCat.org]
[DOI]
(I p)