Difference between revisions of "SpoVM"
| Line 56: | Line 56: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU15810&redirect=T BSU15810] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/spoVM.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/spoVM.html] | ||
| Line 97: | Line 98: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU15810&redirect=T BSU15810] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 13:41, 2 April 2014
- Description: required for normal spore cortex and coat synthesis, inhibits the proteolytic activity of FtsH (adaptor protein)
| Gene name | spoVM |
| Synonyms | |
| Essential | no |
| Product | spore coat morphogenetic protein |
| Function | spore cortex and coat synthesis |
| Gene expression levels in SubtiExpress: spoVM | |
| Interactions involving this protein in SubtInteract: SpoVM | |
| MW, pI | 2 kDa, 11.026 |
| Gene length, protein length | 78 bp, 26 aa |
| Immediate neighbours | yloS, rpmB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 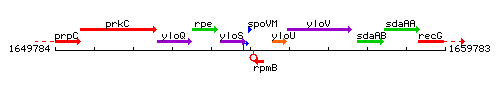
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed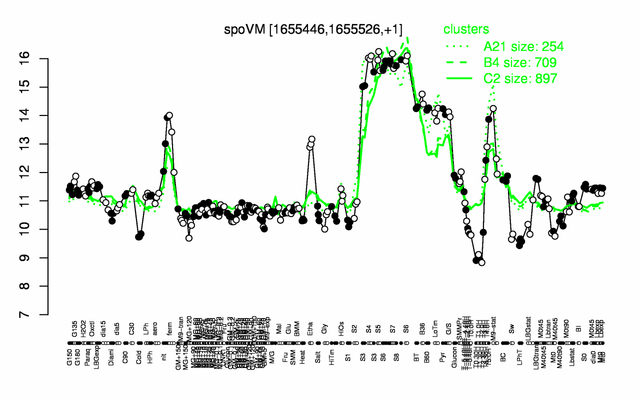
| |
Contents
Categories containing this gene/protein
sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15810
Phenotypes of a mutant
- reduction of sporulation efficiency due to defects in spore coat and cortex assembly PubMed
Database entries
- BsubCyc: BSU15810
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- anchors the basement layer of the spore coat to the surface of the developing spore PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: contains an amphipathic alpha-helix
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU15810
- Structure:
- UniProt: P37817
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: spoVM PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Peter T McKenney, Adam Driks, Patrick Eichenberger
The Bacillus subtilis endospore: assembly and functions of the multilayered coat.
Nat Rev Microbiol: 2013, 11(1);33-44
[PubMed:23202530]
[WorldCat.org]
[DOI]
(I p)
Original publications
Ana Yepes, Johannes Schneider, Benjamin Mielich, Gudrun Koch, Juan-Carlos García-Betancur, Kumaran S Ramamurthi, Hera Vlamakis, Daniel López
The biofilm formation defect of a Bacillus subtilis flotillin-defective mutant involves the protease FtsH.
Mol Microbiol: 2012, 86(2);457-71
[PubMed:22882210]
[WorldCat.org]
[DOI]
(I p)
Sarah E Ebmeier, Irene S Tan, Katie Rose Clapham, Kumaran S Ramamurthi
Small proteins link coat and cortex assembly during sporulation in Bacillus subtilis.
Mol Microbiol: 2012, 84(4);682-96
[PubMed:22463703]
[WorldCat.org]
[DOI]
(I p)
Peter T McKenney, Patrick Eichenberger
Dynamics of spore coat morphogenesis in Bacillus subtilis.
Mol Microbiol: 2012, 83(2);245-60
[PubMed:22171814]
[WorldCat.org]
[DOI]
(I p)
Katherine H Wang, Anabela L Isidro, Lia Domingues, Haig A Eskandarian, Peter T McKenney, Kevin Drew, Paul Grabowski, Ming-Hsiu Chua, Samantha N Barry, Michelle Guan, Richard Bonneau, Adriano O Henriques, Patrick Eichenberger
The coat morphogenetic protein SpoVID is necessary for spore encasement in Bacillus subtilis.
Mol Microbiol: 2009, 74(3);634-49
[PubMed:19775244]
[WorldCat.org]
[DOI]
(I p)
Kumaran S Ramamurthi, Sigolene Lecuyer, Howard A Stone, Richard Losick
Geometric cue for protein localization in a bacterium.
Science: 2009, 323(5919);1354-7
[PubMed:19265022]
[WorldCat.org]
[DOI]
(I p)
Ai Thi Thuy Le, Wolfgang Schumann
Regulation of the spoVM gene of Bacillus subtilis.
Curr Microbiol: 2008, 57(5);484-9
[PubMed:18820968]
[WorldCat.org]
[DOI]
(P p)
Kumaran S Ramamurthi, Katie Rose Clapham, Richard Losick
Peptide anchoring spore coat assembly to the outer forespore membrane in Bacillus subtilis.
Mol Microbiol: 2006, 62(6);1547-57
[PubMed:17427285]
[WorldCat.org]
[DOI]
(P p)
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
Christiaan van Ooij, Richard Losick
Subcellular localization of a small sporulation protein in Bacillus subtilis.
J Bacteriol: 2003, 185(4);1391-8
[PubMed:12562810]
[WorldCat.org]
[DOI]
(P p)
R S Prajapati, T Ogura, S M Cutting
Structural and functional studies on an FtsH inhibitor from Bacillus subtilis.
Biochim Biophys Acta: 2000, 1475(3);353-9
[PubMed:10913836]
[WorldCat.org]
[DOI]
(P p)
K D Price, R Losick
A four-dimensional view of assembly of a morphogenetic protein during sporulation in Bacillus subtilis.
J Bacteriol: 1999, 181(3);781-90
[PubMed:9922240]
[WorldCat.org]
[DOI]
(P p)
S Cutting, M Anderson, E Lysenko, A Page, T Tomoyasu, K Tatematsu, T Tatsuta, L Kroos, T Ogura
SpoVM, a small protein essential to development in Bacillus subtilis, interacts with the ATP-dependent protease FtsH.
J Bacteriol: 1997, 179(17);5534-42
[PubMed:9287010]
[WorldCat.org]
[DOI]
(P p)
P A Levin, N Fan, E Ricca, A Driks, R Losick, S Cutting
An unusually small gene required for sporulation by Bacillus subtilis.
Mol Microbiol: 1993, 9(4);761-71
[PubMed:8231808]
[WorldCat.org]
[DOI]
(P p)