Difference between revisions of "PdhD"
(→Reviews) |
|||
| Line 42: | Line 42: | ||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| − | {{SubtiWiki category|[[carbon core metabolism]]}} | + | {{SubtiWiki category|[[carbon core metabolism]]}}, |
| + | [[most abundant proteins]] | ||
= This gene is a member of the following [[regulons]] = | = This gene is a member of the following [[regulons]] = | ||
| Line 82: | Line 83: | ||
* '''Kinetic information:''' Michaelis-Menten [http://www.ncbi.nlm.nih.gov/pubmed/6414463 PubMed] | * '''Kinetic information:''' Michaelis-Menten [http://www.ncbi.nlm.nih.gov/pubmed/6414463 PubMed] | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' phosphorylated (Ser/Thr/Tyr) [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | * '''Modification:''' phosphorylated (Ser/Thr/Tyr) [http://www.ncbi.nlm.nih.gov/pubmed/17726680 PubMed] | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 117: | Line 118: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pdhD_1531870_1533282_1 pdhD] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=pdhD_1531870_1533282_1 pdhD] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
** ''[[pdhA]]'': [[SigA]] {{PubMed|20081037}} | ** ''[[pdhA]]'': [[SigA]] {{PubMed|20081037}} | ||
** ''[[pdhC]]'': [[SigA]] {{PubMed|11976308}} | ** ''[[pdhC]]'': [[SigA]] {{PubMed|11976308}} | ||
| Line 129: | Line 130: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
=Biological materials = | =Biological materials = | ||
| Line 155: | Line 157: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>12850135 6414463 11976308 17726680 20081037 20933603 24204596</pubmed> | + | <pubmed>12850135 6414463 11976308 17726680 20081037 20933603 24204596 15378759</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:21, 5 March 2014
- Description: dihydrolipoamide dehydrogenase E3 subunit of both pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes
| Gene name | pdhD |
| Synonyms | citL |
| Essential | no |
| Product | dihydrolipoamide dehydrogenase E3 subunit of both pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes |
| Function | links glycolysis and TCA cycle, enzyme in TCA cycle |
| Gene expression levels in SubtiExpress: pdhD | |
| Interactions involving this protein in SubtInteract: PdhD | |
| Metabolic function and regulation of this protein in SubtiPathways: pdhD | |
| MW, pI | 49 kDa, 4.76 |
| Gene length, protein length | 1410 bp, 470 aa |
| Immediate neighbours | pdhC, slp |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 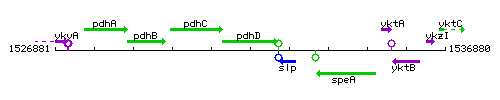
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed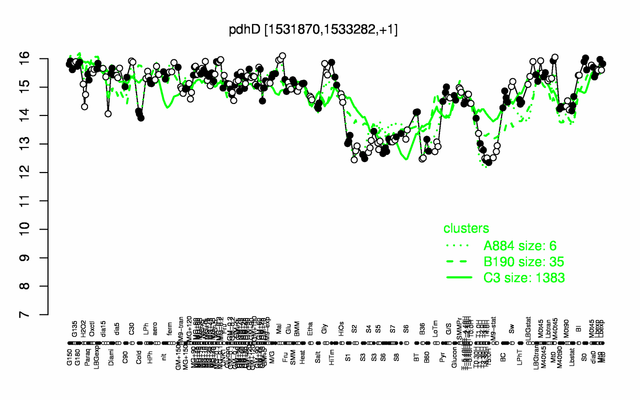
| |
Contents
Categories containing this gene/protein
carbon core metabolism, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14610
Phenotypes of a mutant
- defects in sporulation and unable to grow on glucose as single carbon source PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Protein N(6)-(dihydrolipoyl)lysine + NAD+ = protein N(6)-(lipoyl)lysine + NADH (according to Swiss-Prot)
- Protein family: class-I pyridine nucleotide-disulfide oxidoreductase family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information: Michaelis-Menten PubMed
- Modification: phosphorylated (Ser/Thr/Tyr) PubMed
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 1EBD (complex with binding domain of dihydrolipoamide acetylase, Geobacillus stearothermophilus), 1EBD (complex with binding domain of dihydrolipoamide acetylase, Geobacillus stearothermophilus)
- UniProt: P21880
- KEGG entry: [3]
- E.C. number: 1.8.1.4
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- stringent response: due to presence of guanine at +1 position of the transcript PubMed
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant:
- Expression vector:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Kai Tittmann
Reaction mechanisms of thiamin diphosphate enzymes: redox reactions.
FEBS J: 2009, 276(9);2454-68
[PubMed:19476487]
[WorldCat.org]
[DOI]
(I p)
K F Sheu, J P Blass
The alpha-ketoglutarate dehydrogenase complex.
Ann N Y Acad Sci: 1999, 893;61-78
[PubMed:10672230]
[WorldCat.org]
[DOI]
(P p)
U Neveling, S Bringer-Meyer, H Sahm
Gene and subunit organization of bacterial pyruvate dehydrogenase complexes.
Biochim Biophys Acta: 1998, 1385(2);367-72
[PubMed:9655937]
[WorldCat.org]
[DOI]
(P p)
M S Patel, T E Roche
Molecular biology and biochemistry of pyruvate dehydrogenase complexes.
FASEB J: 1990, 4(14);3224-33
[PubMed:2227213]
[WorldCat.org]
[DOI]
(P p)
P A Frey
Mechanism of coupled electron and group transfer in Escherichia coli pyruvate dehydrogenase.
Ann N Y Acad Sci: 1982, 378;250-64
[PubMed:6805383]
[WorldCat.org]
[DOI]
(P p)
Original publications