Difference between revisions of "SpoIVFA"
| Line 72: | Line 72: | ||
* '''Catalyzed reaction/ biological activity:''' | * '''Catalyzed reaction/ biological activity:''' | ||
| − | * '''Protein family:''' | + | * '''Protein family:''' PDZ protease {{PubMed|24243021}} |
* '''Paralogous protein(s):''' | * '''Paralogous protein(s):''' | ||
| Line 81: | Line 81: | ||
* '''Domains:''' | * '''Domains:''' | ||
| + | ** the ectodomain of [[SpoIVFA]] is cleaved off by [[SpoIVB]] (first cleavage) {{PubMed|24243021}} | ||
| + | ** the domains that inhibits [[SpoIVFB]] is cleaved off by [[CtpB]] (second cleavage) {{PubMed|24243021}} | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 91: | Line 93: | ||
** [[SpoIVFB]]-[[SpoIVFA]], to facilitate the recruitment of [[BofA]] to [[SpoIVFB]] {{PubMed|11959848}} | ** [[SpoIVFB]]-[[SpoIVFA]], to facilitate the recruitment of [[BofA]] to [[SpoIVFB]] {{PubMed|11959848}} | ||
** [[BofA]]-[[SpoIVFA]] {{PubMed|11959848}} | ** [[BofA]]-[[SpoIVFA]] {{PubMed|11959848}} | ||
| + | ** [[SpoIVB]]-[[SpoIVFA]] {{PubMed|24243021}} | ||
| + | ** [[CtpB]]-[[SpoIVFA]] {{PubMed|24243021}} | ||
* '''[[Localization]]:''' | * '''[[Localization]]:''' | ||
| − | ** | + | ** mother cell membrane {{PubMed|24243021}} |
** integral membrane protein {{PubMed|11959848}} | ** integral membrane protein {{PubMed|11959848}} | ||
| Line 107: | Line 111: | ||
=== Additional information=== | === Additional information=== | ||
| − | + | ** the ectodomain of [[SpoIVFA]] is cleaved off by [[SpoIVB]] (first cleavage) {{PubMed|24243021}} | |
| − | cleaved by [[SpoIVB]] | + | ** the domains that inhibits [[SpoIVFB]] is cleaved off by [[CtpB]] (second cleavage) {{PubMed|24243021} |
=Expression and regulation= | =Expression and regulation= | ||
| Line 124: | Line 128: | ||
** [[SpoIIID]]: transcription repression {{PubMed|15383836}} | ** [[SpoIIID]]: transcription repression {{PubMed|15383836}} | ||
| − | * '''Additional information:''' cleaved by [[SpoIVB]] | + | * '''Additional information:''' |
| + | ** the ectodomain of [[SpoIVFA]] is cleaved off by [[SpoIVB]] (first cleavage) {{PubMed|24243021}} | ||
| + | ** the domains that inhibits [[SpoIVFB]] is cleaved off by [[CtpB]] (second cleavage) {{PubMed|24243021}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 10:03, 3 December 2013
- Description: inhibitor of SpoIVFB metalloprotease
| Gene name | spoIVFA |
| Synonyms | bofB, spoVL |
| Essential | no |
| Product | inhibitor of SpoIVFB metalloprotease |
| Function | control of SigK activation |
| Gene expression levels in SubtiExpress: spoIVFA | |
| Interactions involving this protein in SubtInteract: SpoIVFA | |
| MW, pI | 29 kDa, 7.292 |
| Gene length, protein length | 792 bp, 264 aa |
| Immediate neighbours | spoIVFB, minD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 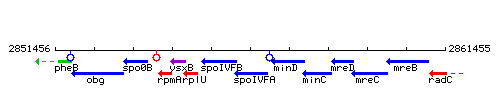
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
sigma factors and their control, sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27980
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: PDZ protease PubMed
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P26936
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References