Difference between revisions of "UvrB"
| Line 86: | Line 86: | ||
* '''[[SubtInteract|Interactions]]:''' | * '''[[SubtInteract|Interactions]]:''' | ||
| − | ** | + | ** [[UvrA]]-[[UvrB]] {{PubMed|16464004}} |
** [[UvrB]]-[[UvrC]] {{PubMed|16464004}} | ** [[UvrB]]-[[UvrC]] {{PubMed|16464004}} | ||
** [[UvrB]] interacts sequentially with [[UvrA]] and [[UvrC]], a complex of the three proteins was never observed {{PubMed|16464004}} | ** [[UvrB]] interacts sequentially with [[UvrA]] and [[UvrC]], a complex of the three proteins was never observed {{PubMed|16464004}} | ||
| + | ** [[UvrB]]-[[PcrA]] {{PubMed|24147116}} | ||
* '''[[Localization]]:''' cytoplasm (according to Swiss-Prot) | * '''[[Localization]]:''' cytoplasm (according to Swiss-Prot) | ||
| Line 141: | Line 142: | ||
<pubmed>16464004 15927210 7801120 22933559 </pubmed> | <pubmed>16464004 15927210 7801120 22933559 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>8226626, 9555905 16426634 21821766</pubmed> | + | <pubmed>8226626, 9555905 16426634 21821766 24147116</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:46, 17 November 2013
- Description: excinuclease ABC (subunit B)
| Gene name | uvrB |
| Synonyms | dinA, uvrA |
| Essential | no |
| Product | excinuclease ABC (subunit B) |
| Function | DNA repair after UV damage |
| Gene expression levels in SubtiExpress: uvrB | |
| Interactions involving this protein in SubtInteract: UvrB | |
| MW, pI | 76 kDa, 5.26 |
| Gene length, protein length | 1983 bp, 661 aa |
| Immediate neighbours | uvrA, csbA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 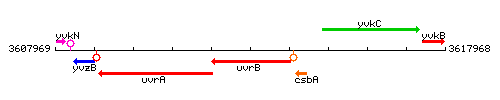
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed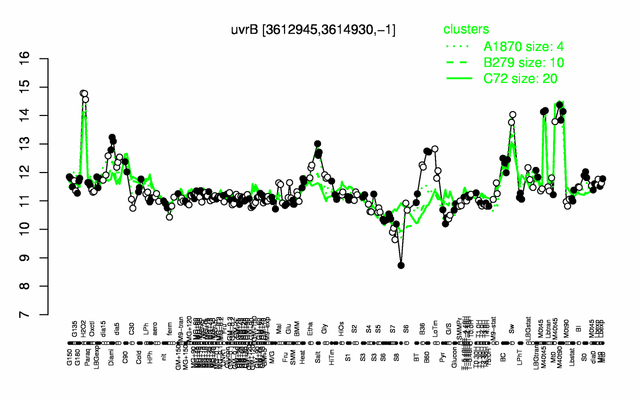
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35170
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
- A mutation was found in this gene after evolution under relaxed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: uvrB family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 2NMV (bound to fluorescein-adducted DNA); 2D7D ternary complex involving UvrB* (a C-terminal truncation of full-length UvrB), a polythymine trinucleotide and ADP PubMed
- UniProt: P37954
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant: GP1175 (del uvrAB::ermC) (available in the Stülke lab)
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Justin S Lenhart, Jeremy W Schroeder, Brian W Walsh, Lyle A Simmons
DNA repair and genome maintenance in Bacillus subtilis.
Microbiol Mol Biol Rev: 2012, 76(3);530-64
[PubMed:22933559]
[WorldCat.org]
[DOI]
(I p)
James J Truglio, Deborah L Croteau, Bennett Van Houten, Caroline Kisker
Prokaryotic nucleotide excision repair: the UvrABC system.
Chem Rev: 2006, 106(2);233-52
[PubMed:16464004]
[WorldCat.org]
[DOI]
(P p)
Bennett Van Houten, Deborah L Croteau, Matthew J DellaVecchia, Hong Wang, Caroline Kisker
'Close-fitting sleeves': DNA damage recognition by the UvrABC nuclease system.
Mutat Res: 2005, 577(1-2);92-117
[PubMed:15927210]
[WorldCat.org]
[DOI]
(P p)
A Sancar
Mechanisms of DNA excision repair.
Science: 1994, 266(5193);1954-6
[PubMed:7801120]
[WorldCat.org]
[DOI]
(P p)
Original publications
Emma J Gwynn, Abigail J Smith, Colin P Guy, Nigel J Savery, Peter McGlynn, Mark S Dillingham
The conserved C-terminus of the PcrA/UvrD helicase interacts directly with RNA polymerase.
PLoS One: 2013, 8(10);e78141
[PubMed:24147116]
[WorldCat.org]
[DOI]
(I e)
Christopher T Brown, Laura K Fishwick, Binna M Chokshi, Marissa A Cuff, Jay M Jackson, Travis Oglesby, Alison T Rioux, Enrique Rodriguez, Gregory S Stupp, Austin H Trupp, James S Woollcombe-Clarke, Tracy N Wright, William J Zaragoza, Jennifer C Drew, Eric W Triplett, Wayne L Nicholson
Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation.
Appl Environ Microbiol: 2011, 77(19);6867-77
[PubMed:21821766]
[WorldCat.org]
[DOI]
(I p)
Jitka Eryilmaz, Simona Ceschini, James Ryan, Stella Geddes, Timothy R Waters, Tracey E Barrett
Structural insights into the cryptic DNA-dependent ATPase activity of UvrB.
J Mol Biol: 2006, 357(1);62-72
[PubMed:16426634]
[WorldCat.org]
[DOI]
(P p)
K W Winterling, D Chafin, J J Hayes, J Sun, A S Levine, R E Yasbin, R Woodgate
The Bacillus subtilis DinR binding site: redefinition of the consensus sequence.
J Bacteriol: 1998, 180(8);2201-11
[PubMed:9555905]
[WorldCat.org]
[DOI]
(P p)
C M Lovett, K C Cho, T M O'Gara
Purification of an SOS repressor from Bacillus subtilis.
J Bacteriol: 1993, 175(21);6842-9
[PubMed:8226626]
[WorldCat.org]
[DOI]
(P p)