Difference between revisions of "ThiL"
Raphael2215 (talk | contribs) |
|||
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 107: | Line 103: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[thiL]]-[[tsaE]]-[[tsaB]]-[[ydiD]]-[[tsaD]]'' {{PubMed|22383849}} |
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=thiL_640662_641639_1 thiL] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=thiL_640662_641639_1 thiL] {{PubMed|22383849}} | ||
| Line 141: | Line 137: | ||
<pubmed>19348578 </pubmed> | <pubmed>19348578 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>16291685,, </pubmed> | + | <pubmed>16291685,22383849, </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:22, 18 October 2012
- Description: thiamine-monophosphate kinase
| Gene name | thiL |
| Synonyms | ydiA, ydxA |
| Essential | no |
| Product | thiamine-monophosphate kinase |
| Function | biosynthesis of thiamine |
| Gene expression levels in SubtiExpress: thiL | |
| Metabolic function and regulation of this protein in SubtiPathways: Thiamin | |
| MW, pI | 35 kDa, 5.036 |
| Gene length, protein length | 975 bp, 325 aa |
| Immediate neighbours | trnE-Asp, ydiB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 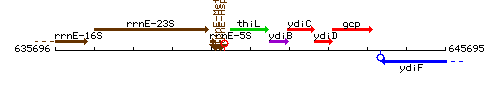
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed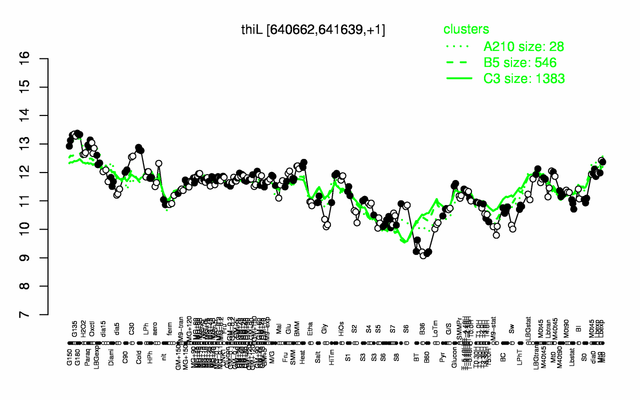
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU05900
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + thiamine phosphate = ADP + thiamine diphosphate (according to Swiss-Prot)
- Protein family: thiamine-monophosphate kinase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1VQV (from Aquifex aeolicus, 33% similarity, 51% identity)
- UniProt: O05514
- KEGG entry: [2]
- E.C. number: 2.7.4.16
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Christopher T Jurgenson, Tadhg P Begley, Steven E Ealick
The structural and biochemical foundations of thiamin biosynthesis.
Annu Rev Biochem: 2009, 78;569-603
[PubMed:19348578]
[WorldCat.org]
[DOI]
(I p)
Original publications
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Ghislain Schyns, Sébastien Potot, Yi Geng, Teresa M Barbosa, Adriano Henriques, John B Perkins
Isolation and characterization of new thiamine-deregulated mutants of Bacillus subtilis.
J Bacteriol: 2005, 187(23);8127-36
[PubMed:16291685]
[WorldCat.org]
[DOI]
(P p)