Difference between revisions of "CggR"
(→Basic information/ Evolution) |
|||
| Line 52: | Line 52: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' transcription repression of the glycolytic ''[[gapA]]'' operon |
* '''Protein family:''' | * '''Protein family:''' | ||
Revision as of 18:03, 14 January 2009
- Description: repressor of the glycolytic gapA operon
| Gene name | cggR |
| Synonyms | yvbQ |
| Essential | no |
| Product | central glycolytic genes regulator |
| Function | transcriptional regulator |
| MW, pI | 37,2 kDa,5.68 |
| Gene length, protein length | 1020 bp, 340 amino acids |
| Immediate neighbours | araE, gapA |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 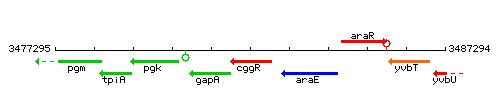
| |
Contents
The gene
Basic information
- Coordinates: 3481786 - 3482805
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription repression of the glycolytic gapA operon
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- DNA binding domain (H-T-H motif) (37–56)
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Swiss prot entry: [3]
- KEGG entry: [4]
Additional information
Expression and regulation
The primary mRNAs of the operon are highly unstable. The primary mRNA is subject to processing at the very end of the cggR open reading frame. This results in stable mature gapA and gapA-pgk-tpiA-pgm-eno mRNAs. The processing event requires the Rny protein.
- Sigma factor: SigA
- Regulatory mechanism: repression
- Database entries: DBTBS
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Your additional remarks
References
- Commichau, F. M., Rothe, F. M., Herzberg, C., Wagner, E., Hellwig, D., Lehnik-Habrink, M., Hammer, E., Völker, U. & Stülke, J. Novel activities of glycolytic enzymes in Bacillus subtilis: Interactions with essential proteins involved in mRNA processing. subm.
- Doan, T., and S. Aymerich. 2003. Regulation of the central glycolytic pathways in Bacillus subtilis: binding of the repressor CggR to its single DNA target sequence is modulated by fructose-1,6-bisphosphate. Mol. Microbiol. 47: 1709-1721. PubMed
- Doan et al. (2008) A phospho-sugar binding domain homologous to NagB enzymes regulates the activity of the central glycolytic genes repressor. Proteins 71:2038-2050. PubMed
- Fillinger, S., Boschi-Muller, S., Azza, S., Dervyn, E., Branlant, G., and Aymerich, S. (2000) Two glyceraldehyde-3-phosphate dehydrogenases with opposite physiological roles in a nonphotosynthetic bacterium. J Biol Chem 275, 14031-14037. PubMed
- Ludwig, H., Homuth, G., Schmalisch, M., Dyka, F. M., Hecker, M., and Stülke, J. (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Mol Microbiol 41, 409-422.PubMed
- Ludwig, H., Rebhan, N., Blencke, H.-M., Merzbacher, M. & Stülke, J. (2002). Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation. Mol Microbiol 45, 543-553.PubMed
- Meinken, C., Blencke, H. M., Ludwig, H., and Stülke, J. (2003) Expression of the glycolytic gapA operon in Bacillus subtilis: differential synthesis of proteins encoded by the operon. Microbiology 149, 751-761. PubMed
- Rezacova et al. (2008) Crystal structures of the effector-binding domain of repressor Central glycolytic gene Regulator from Bacillus subtilis reveal ligand-induced structural changes upon binding of several glycolytic intermediates. Mol. Microbiol. 69:895-910. PubMed
- Zorilla et al. (2007) Fructose-1,6-bisphosphate acts both as an inducer and as a structural cofactor of the central glycolytic genes repressor (CggR). Biochemistry 46:14996-15008. PubMed
- Zorilla et al. (2007) Inducer-modulated cooperative binding of the tetrameric CggR repressor to operator DNA. Biophys. J. 92: 3215-3227. PubMed