Difference between revisions of "NagZ"
(→Extended information on the protein) |
|||
| Line 131: | Line 131: | ||
=References= | =References= | ||
| + | '''Additional references:''' {{PubMed|20826810}} | ||
<pubmed>10627040, 20400549 4628806 4196675</pubmed> | <pubmed>10627040, 20400549 4628806 4196675</pubmed> | ||
| − | + | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 18:22, 27 December 2011
- Description: N-acetylglucosaminidase
| Gene name | nagZ |
| Synonyms | yzbA, ybbD |
| Essential | no |
| Product | N-acetylglucosaminidase |
| Function | cell wall recycling |
| Metabolic function and regulation of this protein in SubtiPathways: Murein recycling | |
| MW, pI | 70 kDa, 9.76 |
| Gene length, protein length | 1926 bp, 642 aa |
| Immediate neighbours | ybbC, amiE |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 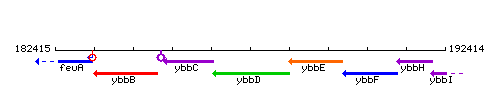
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01660
Phenotypes of a mutant
increased autolysis PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: cleaves muropeptides derived from peptidoglycan, but not peptidoglycan itself PubMed
- Protein family: glycosyl hydrolase 3 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- secreted (with signal peptide), remains to some extent cell wall-associated PubMed
Database entries
- UniProt: P40406
- KEGG entry: [2]
- E.C. number:
Additional information
The gene is mis-annotated in KEGG as an ortholog of beta-N-acetylhexosaminidase EC 3.2.1.52. It is marked in MetaCyc as “similar to beta-hexosaminidase”. No EC annotation is available in Swiss-ProtSwiss-Prot.supporting the annotation is available. PubMed
Expression and regulation
- Operon:
- Regulation:
- expressed in late exponential and early stationary phase PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: available in Christoph Mayer's lab PubMed
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional references: PubMed
Silke Litzinger, Amanda Duckworth, Katja Nitzsche, Christian Risinger, Valentin Wittmann, Christoph Mayer
Muropeptide rescue in Bacillus subtilis involves sequential hydrolysis by beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase.
J Bacteriol: 2010, 192(12);3132-43
[PubMed:20400549]
[WorldCat.org]
[DOI]
(I p)
Jonathan Reizer, Steffi Bachem, Aiala Reizer, Maryvonne Arnaud, Milton H Saier, Jörg Stülke
Novel phosphotransferase system genes revealed by genome analysis - the complete complement of PTS proteins encoded within the genome of Bacillus subtilis.
Microbiology (Reading): 1999, 145 ( Pt 12);3419-3429
[PubMed:10627040]
[WorldCat.org]
[DOI]
(P p)
R C Berkeley, S J Brewer, J M Ortiz, J B Gillespie
An exo- -N-acetylglucosaminidase from Bacillus subtilis B; characterization.
Biochim Biophys Acta: 1973, 309(1);157-68
[PubMed:4196675]
[WorldCat.org]
[DOI]
(P p)
J M Ortiz, J B Gillespie, R C Berkeley
An exo- -N-acetylglucosaminidase from Bacillus subtilis B; extraction and purification.
Biochim Biophys Acta: 1972, 289(1);174-86
[PubMed:4628806]
[WorldCat.org]
[DOI]
(P p)