Difference between revisions of "Dxs"
(→Original publications) |
|||
| Line 136: | Line 136: | ||
<pubmed>19348578 </pubmed> | <pubmed>19348578 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>12882400,17135236 , </pubmed> | + | <pubmed>12882400,17135236 , 21296950 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:33, 8 February 2011
- Description: 1-deoxyxylulose-5-phosphate synthase
| Gene name | dxs |
| Synonyms | yqiE |
| Essential | yes PubMed |
| Product | 1-deoxyxylulose-5-phosphate synthase |
| Function | biosynthesis of thiamine |
| Metabolic function and regulation of this protein in SubtiPathways: Thiamin | |
| MW, pI | 69 kDa, 6.002 |
| Gene length, protein length | 1899 bp, 633 aa |
| Immediate neighbours | yqxC, yqiD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 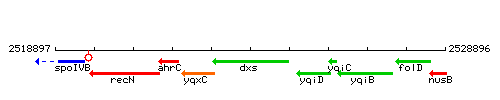
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
biosynthesis of cofactors, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU24270
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Pyruvate + D-glyceraldehyde 3-phosphate = 1-deoxy-D-xylulose 5-phosphate + CO2 (according to Swiss-Prot)
- Protein family: DXPS subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- UniProt: P54523
- KEGG entry: [2]
- E.C. number: 2.2.1.7
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Junfeng Xue, Birgitte K Ahring
Enhancing isoprene production by genetic modification of the 1-deoxy-d-xylulose-5-phosphate pathway in Bacillus subtilis.
Appl Environ Microbiol: 2011, 77(7);2399-405
[PubMed:21296950]
[WorldCat.org]
[DOI]
(I p)
Song Xiang, Gerlinde Usunow, Gudrun Lange, Marco Busch, Liang Tong
Crystal structure of 1-deoxy-D-xylulose 5-phosphate synthase, a crucial enzyme for isoprenoids biosynthesis.
J Biol Chem: 2007, 282(4);2676-82
[PubMed:17135236]
[WorldCat.org]
[DOI]
(P p)
Akihiro Sakai, Natsumi Kinoshita, Makoto Kita, Tohoru Katsuragi, Yoshiki Tani
Investigation of 1-deoxy-D-xylulose 5-phosphate synthase and transketolase of Bacillus subtilis in relation to vitamin B6 biosynthesis.
J Nutr Sci Vitaminol (Tokyo): 2003, 49(1);73-5
[PubMed:12882400]
[WorldCat.org]
[DOI]
(P p)