Difference between revisions of "Nos"
(→Expression and regulation) |
|||
| Line 66: | Line 66: | ||
* '''Modification:''' | * '''Modification:''' | ||
| − | * '''Cofactor(s):''' | + | * '''Cofactor(s):''' heme {{PubMed|20006999}} |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 118: | Line 118: | ||
=References= | =References= | ||
| − | <pubmed>12220171,11856757,, </pubmed> | + | <pubmed>12220171,11856757,20006999 , </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 05:38, 18 December 2009
- Description: nitric-oxide synthase
| Gene name | nos |
| Synonyms | yflM |
| Essential | no |
| Product | nitric-oxide synthase |
| Function | production of nitric oxide |
| MW, pI | 38 kDa, 5.528 |
| Gene length, protein length | 1008 bp, 336 aa |
| Immediate neighbours | yflN, yflL |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 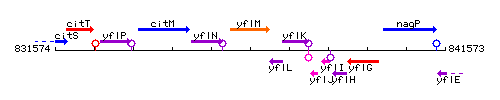
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU07630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: Bacterial NOS oxygenase subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): heme PubMed
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- UniProt: O34453
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information: Note that the gene yflL is located between nos and yflK, but on the opposite strand.
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Zhi-Qiang Wang, Chin-Chuan Wei, Dennis J Stuehr
How does a valine residue that modulates heme-NO binding kinetics in inducible NO synthase regulate enzyme catalysis?
J Inorg Biochem: 2010, 104(3);349-56
[PubMed:20006999]
[WorldCat.org]
[DOI]
(I p)
Kartikeya Pant, Alexandrine M Bilwes, Subrata Adak, Dennis J Stuehr, Brian R Crane
Structure of a nitric oxide synthase heme protein from Bacillus subtilis.
Biochemistry: 2002, 41(37);11071-9
[PubMed:12220171]
[WorldCat.org]
[DOI]
(P p)
Subrata Adak, Kulwant S Aulak, Dennis J Stuehr
Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis.
J Biol Chem: 2002, 277(18);16167-71
[PubMed:11856757]
[WorldCat.org]
[DOI]
(P p)