FapR
- Description: repressor of fatty acid synthetic genes
| Gene name | fapR |
| Synonyms | ylpC |
| Essential | no |
| Product | transcriptional repressor |
| Function | regulation of fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: fapR | |
| Interactions involving this protein in SubtInteract: FapR | |
| Metabolic function and regulation of this protein in SubtiPathways: fapR | |
| MW, pI | 21 kDa, 5.393 |
| Gene length, protein length | 564 bp, 188 aa |
| Immediate neighbours | recG, plsX |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 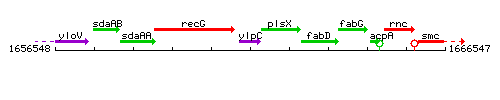
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed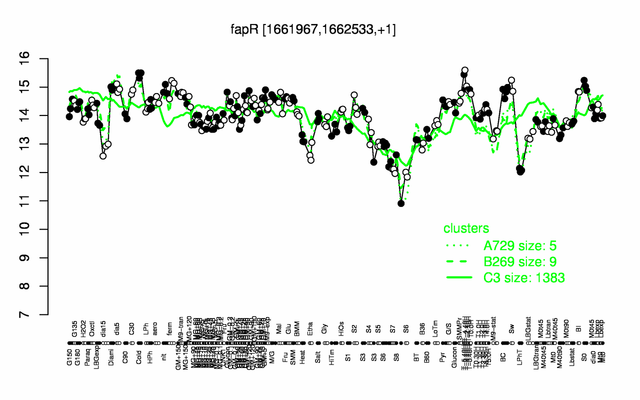
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, transcription factors and their control
This gene is a member of the following regulons
The FapR regulon
The gene
Basic information
- Locus tag: BSU15880
Phenotypes of a mutant
Database entries
- BsubCyc: BSU15880
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: FapR regulates the expression of at least 10 genes (the fap regulon). It autoregulates its own expression. Malonyl-CoA, a precursor of fatty acid biosynthesis, binds to FapR changing its conformation to a non-DNA binding state. Hence, conditions that cause malonyl-CoA accumulation, like fatty acid biosynthesis inhibition, derepress the fap regulon.
- Protein family: fapR family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity: malonyl-CoA and malonyl-ACP act as the molecular inducer of the FapR regulon PubMed
Database entries
- BsubCyc: BSU15880
- UniProt: O34835
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 85 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 490 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 412 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 729 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Yasutaro Fujita, Hiroshi Matsuoka, Kazutake Hirooka
Regulation of fatty acid metabolism in bacteria.
Mol Microbiol: 2007, 66(4);829-39
[PubMed:17919287]
[WorldCat.org]
[DOI]
(P p)
The FapR regulon
Other original publications
Additional publications: PubMed
Mariano A Martinez, María-Eugenia Zaballa, Francis Schaeffer, Marco Bellinzoni, Daniela Albanesi, Gustavo E Schujman, Alejandro J Vila, Pedro M Alzari, Diego de Mendoza
A novel role of malonyl-ACP in lipid homeostasis.
Biochemistry: 2010, 49(14);3161-7
[PubMed:20201588]
[WorldCat.org]
[DOI]
(I p)
Mariano A Martinez, Diego de Mendoza, Gustavo E Schujman
Transcriptional and functional characterization of the gene encoding acyl carrier protein in Bacillus subtilis.
Microbiology (Reading): 2010, 156(Pt 2);484-495
[PubMed:19850612]
[WorldCat.org]
[DOI]
(I p)
Yong-Mei Zhang, Charles O Rock
Transcriptional regulation in bacterial membrane lipid synthesis.
J Lipid Res: 2009, 50 Suppl(Suppl);S115-9
[PubMed:18941141]
[WorldCat.org]
[DOI]
(P p)
Letal I Salzberg, John D Helmann
Phenotypic and transcriptomic characterization of Bacillus subtilis mutants with grossly altered membrane composition.
J Bacteriol: 2008, 190(23);7797-807
[PubMed:18820022]
[WorldCat.org]
[DOI]
(I p)
Gustavo E Schujman, Silvia Altabe, Diego de Mendoza
A malonyl-CoA-dependent switch in the bacterial response to a dysfunction of lipid metabolism.
Mol Microbiol: 2008, 68(4);987-96
[PubMed:18384517]
[WorldCat.org]
[DOI]
(I p)
Gustavo E Schujman, Marcelo Guerin, Alejandro Buschiazzo, Francis Schaeffer, Leticia I Llarrull, Georgina Reh, Alejandro J Vila, Pedro M Alzari, Diego de Mendoza
Structural basis of lipid biosynthesis regulation in Gram-positive bacteria.
EMBO J: 2006, 25(17);4074-83
[PubMed:16932747]
[WorldCat.org]
[DOI]
(P p)
Natalia Comella, Alan D Grossman
Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis.
Mol Microbiol: 2005, 57(4);1159-74
[PubMed:16091051]
[WorldCat.org]
[DOI]
(P p)