Difference between revisions of "TuaD"
| Line 29: | Line 29: | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
|- | |- | ||
| − | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=tuaD_3654139_3655524_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:tuaD_expression.png|500px]] | + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=tuaD_3654139_3655524_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:tuaD_expression.png|500px|link=http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU35580]] |
|- | |- | ||
|} | |} | ||
Revision as of 14:43, 16 May 2013
- Description: UDP glucose 6-dehydrogenase
| Gene name | tuaD |
| Synonyms | yvhD |
| Essential | no |
| Product | UDP glucose 6-dehydrogenase |
| Function | biosynthesis of teichuronic acid |
| Gene expression levels in SubtiExpress: tuaD | |
| Interactions involving this protein in SubtInteract: TuaD | |
| MW, pI | 49 kDa, 6.107 |
| Gene length, protein length | 1383 bp, 461 aa |
| Immediate neighbours | tuaE, tuaC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed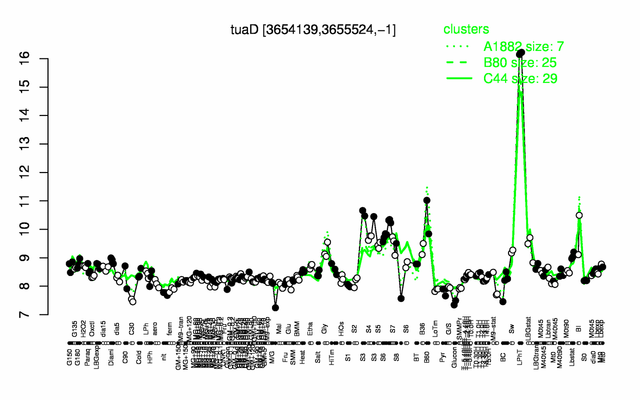
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35580
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: UDP-glucose + 2 NAD+ + H2O = UDP-glucuronate + 2 NADH (according to Swiss-Prot)
- Protein family: UDP-glucose/GDP-mannose dehydrogenase family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O32271
- KEGG entry: [3]
- E.C. number: 1.1.1.22
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ivan Mijakovic, Lucia Musumeci, Lutz Tautz, Dina Petranovic, Robert A Edwards, Peter Ruhdal Jensen, Tomas Mustelin, Josef Deutscher, Nunzio Bottini
In vitro characterization of the Bacillus subtilis protein tyrosine phosphatase YwqE.
J Bacteriol: 2005, 187(10);3384-90
[PubMed:15866923]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Sandrine Poncet, Grégory Boël, Alain Mazé, Sylvie Gillet, Emmanuel Jamet, Paulette Decottignies, Christophe Grangeasse, Patricia Doublet, Pierre Le Maréchal, Josef Deutscher
Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases.
EMBO J: 2003, 22(18);4709-18
[PubMed:12970183]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, C Scharf, M Hecker
Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis.
J Bacteriol: 2000, 182(16);4478-90
[PubMed:10913081]
[WorldCat.org]
[DOI]
(P p)
Maryam Lahooti, Colin R Harwood
Transcriptional analysis of the Bacillus subtilis teichuronic acid operon.
Microbiology (Reading): 1999, 145 ( Pt 12);3409-3417
[PubMed:10627039]
[WorldCat.org]
[DOI]
(P p)
Marco Pagni, Vladimir Lazarevic, Blazenka Soldo, Dimitri Karamata
Assay for UDPglucose 6-dehydrogenase in phosphate-starved cells: gene tuaD of Bacillus subtilis 168 encodes the UDPglucose 6-dehydrogenase involved in teichuronic acid synthesis.
Microbiology (Reading): 1999, 145 ( Pt 5);1049-1053
[PubMed:10376820]
[WorldCat.org]
[DOI]
(P p)
B Soldo, V Lazarevic, M Pagni, D Karamata
Teichuronic acid operon of Bacillus subtilis 168.
Mol Microbiol: 1999, 31(3);795-805
[PubMed:10048024]
[WorldCat.org]
[DOI]
(P p)
Wei Liu, F Marion Hulett
Comparison of PhoP binding to the tuaA promoter with PhoP binding to other Pho-regulon promoters establishes a Bacillus subtilis Pho core binding site.
Microbiology (Reading): 1998, 144 ( Pt 5);1443-1450
[PubMed:9611818]
[WorldCat.org]
[DOI]
(P p)