RpoE
- Description: RNA polymerase delta subunit, affects the regulation of RNA polymerase by the concentration of the initiating nucleoside triphosphate ([iNTP])
| Gene name | rpoE |
| Synonyms | |
| Essential | no |
| Product | RNA polymerase delta subunit |
| Function | transcription |
| Gene expression levels in SubtiExpress: rpoE | |
| Interactions involving this protein in SubtInteract: RpoE | |
| Metabolic function and regulation of this protein in SubtiPathways: Fatty acid degradation | |
| MW, pI | 20 kDa, 3.654 |
| Gene length, protein length | 519 bp, 173 aa |
| Immediate neighbours | pyrG, acdA |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 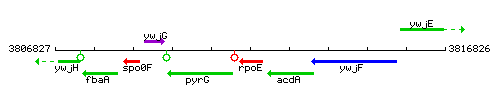
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed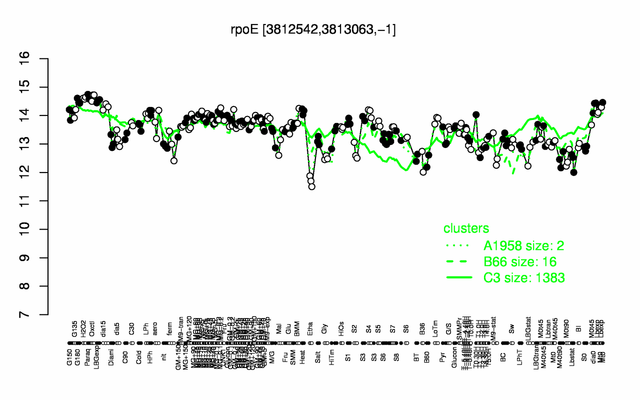
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU37160
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: rpoE family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- closely associated to RNA polymerase (RpoA(2)-RpoB-RpoC) PubMed
- Localization:
- closely associated with RNA polymerase involved in transcribing both mRNA and rRNA operons PubMed
Database entries
- UniProt: P12464
- KEGG entry: [3]
- E.C. number: 2.7.7.6
Additional information
Expression and regulation
- Additional information:
- present at equimolar levels with RNA polymerase PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Arthur Aronson, Purdue University, West Lafayette, USA homepage
Your additional remarks
References
Reviews
Lakshminarayan M Iyer, L Aravind
Insights from the architecture of the bacterial transcription apparatus.
J Struct Biol: 2012, 179(3);299-319
[PubMed:22210308]
[WorldCat.org]
[DOI]
(I p)
Original publications
Alžbeta Rabatinová, Hana Šanderová, Jitka Jirát Matějčková, Jana Korelusová, Luděk Sojka, Ivan Barvík, Veronika Papoušková, Vladimír Sklenár, Lukáš Žídek, Libor Krásný
The δ subunit of RNA polymerase is required for rapid changes in gene expression and competitive fitness of the cell.
J Bacteriol: 2013, 195(11);2603-11
[PubMed:23543716]
[WorldCat.org]
[DOI]
(I p)
Anna Zawadzka-Kazimierczuk, Wiktor Koźmiński, Hana Sanderová, Libor Krásný
High dimensional and high resolution pulse sequences for backbone resonance assignment of intrinsically disordered proteins.
J Biomol NMR: 2012, 52(4);329-37
[PubMed:22350953]
[WorldCat.org]
[DOI]
(I p)
Jiří Nováček, Anna Zawadzka-Kazimierczuk, Veronika Papoušková, Lukáš Zídek, Hana Sanderová, Libor Krásný, Wiktor Koźmiński, Vladimír Sklenář
5D 13C-detected experiments for backbone assignment of unstructured proteins with a very low signal dispersion.
J Biomol NMR: 2011, 50(1);1-11
[PubMed:21424579]
[WorldCat.org]
[DOI]
(I p)
Veronika Motáčková, Jiří Nováček, Anna Zawadzka-Kazimierczuk, Krzysztof Kazimierczuk, Lukáš Zídek, Hana Sanderová, Libor Krásný, Wiktor Koźmiński, Vladimír Sklenář
Strategy for complete NMR assignment of disordered proteins with highly repetitive sequences based on resolution-enhanced 5D experiments.
J Biomol NMR: 2010, 48(3);169-77
[PubMed:20890634]
[WorldCat.org]
[DOI]
(I p)
Geoff P Doherty, Mark J Fogg, Anthony J Wilkinson, Peter J Lewis
Small subunits of RNA polymerase: localization, levels and implications for core enzyme composition.
Microbiology (Reading): 2010, 156(Pt 12);3532-3543
[PubMed:20724389]
[WorldCat.org]
[DOI]
(I p)
Veronika Motácková, Hana Sanderová, Lukás Zídek, Jirí Novácek, Petr Padrta, Alzbeta Svenková, Jana Korelusová, Jirí Jonák, Libor Krásný, Vladimír Sklenár
Solution structure of the N-terminal domain of Bacillus subtilis delta subunit of RNA polymerase and its classification based on structural homologs.
Proteins: 2010, 78(7);1807-10
[PubMed:20310067]
[WorldCat.org]
[DOI]
(I p)
Hiroshi Matsuoka, Kazutake Hirooka, Yasutaro Fujita
Organization and function of the YsiA regulon of Bacillus subtilis involved in fatty acid degradation.
J Biol Chem: 2007, 282(8);5180-94
[PubMed:17189250]
[WorldCat.org]
[DOI]
(P p)
F J López de Saro, N Yoshikawa, J D Helmann
Expression, abundance, and RNA polymerase binding properties of the delta factor of Bacillus subtilis.
J Biol Chem: 1999, 274(22);15953-8
[PubMed:10336502]
[WorldCat.org]
[DOI]
(P p)
F J López de Saro, A Y Woody, J D Helmann
Structural analysis of the Bacillus subtilis delta factor: a protein polyanion which displaces RNA from RNA polymerase.
J Mol Biol: 1995, 252(2);189-202
[PubMed:7545758]
[WorldCat.org]
[DOI]
(P p)
Y L Juang, J D Helmann
Pathway of promoter melting by Bacillus subtilis RNA polymerase at a stable RNA promoter: effects of temperature, delta protein, and sigma factor mutations.
Biochemistry: 1995, 34(26);8465-73
[PubMed:7599136]
[WorldCat.org]
[DOI]
(P p)
Y L Juang, J D Helmann
The delta subunit of Bacillus subtilis RNA polymerase. An allosteric effector of the initiation and core-recycling phases of transcription.
J Mol Biol: 1994, 239(1);1-14
[PubMed:7515111]
[WorldCat.org]
[DOI]
(P p)
M Lampe, C Binnie, R Schmidt, R Losick
Cloned gene encoding the delta subunit of Bacillus subtilis RNA polymerase.
Gene: 1988, 67(1);13-9
[PubMed:2843435]
[WorldCat.org]
[DOI]
(P p)
E I Hyde, M D Hilton, H R Whiteley
Interactions of Bacillus subtilis RNA polymerase with subunits determining the specificity of initiation. Sigma and delta peptides can bind simultaneously to core.
J Biol Chem: 1986, 261(35);16565-70
[PubMed:3097010]
[WorldCat.org]
(P p)
E C Achberger, H R Whiteley
The role of the delta peptide of the Bacillus subtilis RNA polymerase in promoter selection.
J Biol Chem: 1981, 256(14);7424-32
[PubMed:6788769]
[WorldCat.org]
(P p)