RplV
- Description: ribosomal protein
| Gene name | rplV |
| Synonyms | |
| Essential | no PubMed |
| Product | ribosomal protein L22 (BL17) |
| Function | translation |
| Gene expression levels in SubtiExpress: rplV | |
| Interactions involving this protein in SubtInteract: RplV | |
| MW, pI | 12 kDa, 11.225 |
| Gene length, protein length | 339 bp, 113 aa |
| Immediate neighbours | rpsS, rpsC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 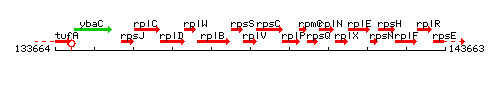
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
translation, universally conserved proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01210
Phenotypes of a mutant
Database entries
- BsubCyc: BSU01210
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ribosomal protein L22P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU01210
- Structure:
- UniProt: P42060
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon: rpsJ-rplC-rplD-rplW-rplB-rpsS-rplV-rpsC-rplP-rpmC-rpsQ-rplN-rplX-rplE-rpsN-rpsH-rplF-rplR-rpsE-rpmD-rplO PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Genki Akanuma, Hideaki Nanamiya, Yousuke Natori, Koichi Yano, Shota Suzuki, Shuya Omata, Morio Ishizuka, Yasuhiko Sekine, Fujio Kawamura
Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation.
J Bacteriol: 2012, 194(22);6282-91
[PubMed:23002217]
[WorldCat.org]
[DOI]
(I p)
Matthew A Lauber, William E Running, James P Reilly
B. subtilis ribosomal proteins: structural homology and post-translational modifications.
J Proteome Res: 2009, 8(9);4193-206
[PubMed:19653700]
[WorldCat.org]
[DOI]
(P p)
Rouven Bingel-Erlenmeyer, Rebecca Kohler, Günter Kramer, Arzu Sandikci, Snjezana Antolić, Timm Maier, Christiane Schaffitzel, Brigitte Wiedmann, Bernd Bukau, Nenad Ban
A peptide deformylase-ribosome complex reveals mechanism of nascent chain processing.
Nature: 2008, 452(7183);108-11
[PubMed:18288106]
[WorldCat.org]
[DOI]
(I p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
X Li, L Lindahl, Y Sha, J M Zengel
Analysis of the Bacillus subtilis S10 ribosomal protein gene cluster identifies two promoters that may be responsible for transcription of the entire 15-kilobase S10-spc-alpha cluster.
J Bacteriol: 1997, 179(22);7046-54
[PubMed:9371452]
[WorldCat.org]
[DOI]
(P p)
J W Suh, S A Boylan, S H Oh, C W Price
Genetic and transcriptional organization of the Bacillus subtilis spc-alpha region.
Gene: 1996, 169(1);17-23
[PubMed:8635744]
[WorldCat.org]
[DOI]
(P p)