BcrC
- Description: undecaprenyl pyrophosphate phosphatase, bacitracin resistance
| Gene name | bcrC |
| Synonyms | ywoA |
| Essential | no |
| Product | undecaprenyl pyrophosphate phosphatase |
| Function | resistance to bacitracin and oxidative stress |
| Gene expression levels in SubtiExpress: bcrC | |
| MW, pI | 21 kDa, 9.455 |
| Gene length, protein length | 579 bp, 193 aa |
| Immediate neighbours | nrgB, ywnJ |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 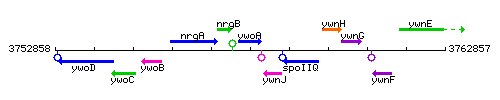
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed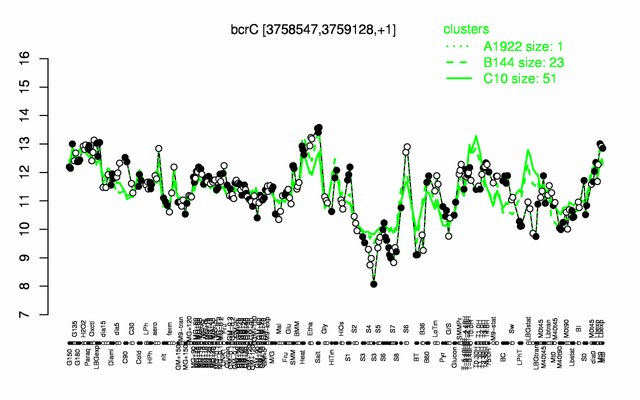
| |
Contents
Categories containing this gene/protein
cell envelope stress proteins (controlled by SigM, V, W, X, Y), heat shock proteins, resistance against toxins/ antibiotics, membrane proteins
This gene is a member of the following regulons
SigI regulon, SigM regulon, SigW regulon, SigX regulon
The gene
Basic information
- Locus tag: BSU36530
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: bcrC/ybjG/ywoA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: P94571
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon: bcrC
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Takashi Inaoka, Kozo Ochi
Undecaprenyl pyrophosphate involvement in susceptibility of Bacillus subtilis to rare earth elements.
J Bacteriol: 2012, 194(20);5632-7
[PubMed:22904278]
[WorldCat.org]
[DOI]
(I p)
Chi-Ling Tseng, Gwo-Chyuan Shaw
Genetic evidence for the actin homolog gene mreBH and the bacitracin resistance gene bcrC as targets of the alternative sigma factor SigI of Bacillus subtilis.
J Bacteriol: 2008, 190(5);1561-7
[PubMed:18156261]
[WorldCat.org]
[DOI]
(I p)
Adrian J Jervis, Penny D Thackray, Chris W Houston, Malcolm J Horsburgh, Anne Moir
SigM-responsive genes of Bacillus subtilis and their promoters.
J Bacteriol: 2007, 189(12);4534-8
[PubMed:17434969]
[WorldCat.org]
[DOI]
(P p)
Min Cao, Charles M Moore, John D Helmann
Bacillus subtilis paraquat resistance is directed by sigmaM, an extracytoplasmic function sigma factor, and is conferred by YqjL and BcrC.
J Bacteriol: 2005, 187(9);2948-56
[PubMed:15838020]
[WorldCat.org]
[DOI]
(P p)
Thorsten Mascher, Neil G Margulis, Tao Wang, Rick W Ye, John D Helmann
Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon.
Mol Microbiol: 2003, 50(5);1591-604
[PubMed:14651641]
[WorldCat.org]
[DOI]
(P p)
Remi Bernard, Pascale Joseph, Annick Guiseppi, Marc Chippaux, François Denizot
YtsCD and YwoA, two independent systems that confer bacitracin resistance to Bacillus subtilis.
FEMS Microbiol Lett: 2003, 228(1);93-7
[PubMed:14612242]
[WorldCat.org]
[DOI]
(P p)
Reiko Ohki, Kozue Tateno, Youji Okada, Haruo Okajima, Kei Asai, Yoshito Sadaie, Makiko Murata, Toshiko Aiso
A bacitracin-resistant Bacillus subtilis gene encodes a homologue of the membrane-spanning subunit of the Bacillus licheniformis ABC transporter.
J Bacteriol: 2003, 185(1);51-9
[PubMed:12486040]
[WorldCat.org]
[DOI]
(P p)
Min Cao, John D Helmann
Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function sigma factors.
J Bacteriol: 2002, 184(22);6123-9
[PubMed:12399481]
[WorldCat.org]
[DOI]
(P p)