YrvO
- Description: cysteine desulfurase involved in 2-thiouridine (s2U) modification of the wobble position in glutamate, glutamine and lysine tRNA molecules
| Gene name | yrvO |
| Synonyms | nifS, iscS |
| Essential | yes PubMed |
| Product | cysteine desulfurase |
| Function | tRNA modification |
| Gene expression levels in SubtiExpress: yrvO | |
| MW, pI | 41 kDa, 5.463 |
| Gene length, protein length | 1137 bp, 379 aa |
| Immediate neighbours | trmU, cymR |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 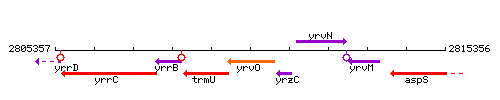
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
essential genes, translation, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27510
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU27510
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-385 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU27510
- UniProt: O34599
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Katherine A Black, Patricia C Dos Santos
Abbreviated Pathway for Biosynthesis of 2-Thiouridine in Bacillus subtilis.
J Bacteriol: 2015, 197(11);1952-62
[PubMed:25825430]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Dorothea Kessler
Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes.
FEMS Microbiol Rev: 2006, 30(6);825-40
[PubMed:17064282]
[WorldCat.org]
[DOI]
(P p)
Soon-Yong Choi, Dindo Reyes, Montira Leelakriangsak, Peter Zuber
The global regulator Spx functions in the control of organosulfur metabolism in Bacillus subtilis.
J Bacteriol: 2006, 188(16);5741-51
[PubMed:16885442]
[WorldCat.org]
[DOI]
(P p)
K Kobayashi, S D Ehrlich, A Albertini, G Amati, K K Andersen, M Arnaud, K Asai, S Ashikaga, S Aymerich, P Bessieres, F Boland, S C Brignell, S Bron, K Bunai, J Chapuis, L C Christiansen, A Danchin, M Débarbouille, E Dervyn, E Deuerling, K Devine, S K Devine, O Dreesen, J Errington, S Fillinger, S J Foster, Y Fujita, A Galizzi, R Gardan, C Eschevins, T Fukushima, K Haga, C R Harwood, M Hecker, D Hosoya, M F Hullo, H Kakeshita, D Karamata, Y Kasahara, F Kawamura, K Koga, P Koski, R Kuwana, D Imamura, M Ishimaru, S Ishikawa, I Ishio, D Le Coq, A Masson, C Mauël, R Meima, R P Mellado, A Moir, S Moriya, E Nagakawa, H Nanamiya, S Nakai, P Nygaard, M Ogura, T Ohanan, M O'Reilly, M O'Rourke, Z Pragai, H M Pooley, G Rapoport, J P Rawlins, L A Rivas, C Rivolta, A Sadaie, Y Sadaie, M Sarvas, T Sato, H H Saxild, E Scanlan, W Schumann, J F M L Seegers, J Sekiguchi, A Sekowska, S J Séror, M Simon, P Stragier, R Studer, H Takamatsu, T Tanaka, M Takeuchi, H B Thomaides, V Vagner, J M van Dijl, K Watabe, A Wipat, H Yamamoto, M Yamamoto, Y Yamamoto, K Yamane, K Yata, K Yoshida, H Yoshikawa, U Zuber, N Ogasawara
Essential Bacillus subtilis genes.
Proc Natl Acad Sci U S A: 2003, 100(8);4678-83
[PubMed:12682299]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
J T Kaiser, T Clausen, G P Bourenkow, H D Bartunik, S Steinbacher, R Huber
Crystal structure of a NifS-like protein from Thermotoga maritima: implications for iron sulphur cluster assembly.
J Mol Biol: 2000, 297(2);451-64
[PubMed:10715213]
[WorldCat.org]
[DOI]
(P p)