YheA
- Description: unknown
| Gene name | yheA |
| Synonyms | |
| Essential | no |
| Product | unknown |
| Function | unknown |
| Gene expression levels in SubtiExpress: yheA | |
| MW, pI | 13 kDa, 4.415 |
| Gene length, protein length | 351 bp, 117 aa |
| Immediate neighbours | yheB, yhaZ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 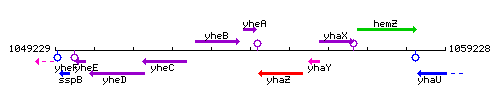
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
proteins of unknown function, phosphoproteins, sporulation proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU09800
Phenotypes of a mutant
Database entries
- BsubCyc: BSU09800
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: UPF0342 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-51 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU09800
- Structure: 2OEE
- UniProt: O07542
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 1148 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 7716 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 44900 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 17904 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 13778 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References