Tgt
- Description: tRNA-guanine transglycosylase
| Gene name | tgt |
| Synonyms | |
| Essential | no |
| Product | tRNA-guanine transglycosylase |
| Function | tRNA modification |
| Gene expression levels in SubtiExpress: tgt | |
| MW, pI | 43 kDa, 6.149 |
| Gene length, protein length | 1143 bp, 381 aa |
| Immediate neighbours | yrbF, queA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 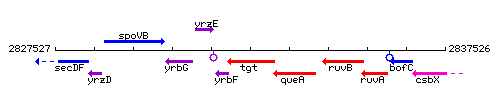
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed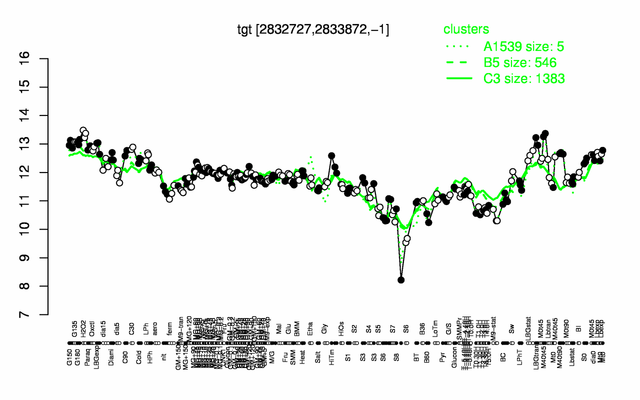
| |
Contents
Categories containing this gene/protein
translation, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27710
Phenotypes of a mutant
Database entries
- BsubCyc: BSU27710
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: [tRNA]-guanine + queuine = [tRNA]-queuine + guanine (according to Swiss-Prot)
- Protein family: queuine tRNA-ribosyltransferase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU27710
- Structure:
- UniProt: O32053
- KEGG entry: [2]
- E.C. number: 2.4.2.29
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ruth Brenk, Milton T Stubbs, Andreas Heine, Klaus Reuter, Gerhard Klebe
Flexible adaptations in the structure of the tRNA-modifying enzyme tRNA-guanine transglycosylase and their implications for substrate selectivity, reaction mechanism and structure-based drug design.
Chembiochem: 2003, 4(10);1066-77
[PubMed:14523925]
[WorldCat.org]
[DOI]
(P p)
Wei Xie, Xianjun Liu, Raven H Huang
Chemical trapping and crystal structure of a catalytic tRNA guanine transglycosylase covalent intermediate.
Nat Struct Biol: 2003, 10(10);781-8
[PubMed:12949492]
[WorldCat.org]
[DOI]
(P p)
DeeAnne M Goodenough-Lashua, George A Garcia
tRNA-guanine transglycosylase from E. coli: a ping-pong kinetic mechanism is consistent with nucleophilic catalysis.
Bioorg Chem: 2003, 31(4);331-44
[PubMed:12877882]
[WorldCat.org]
[DOI]
(P p)
G A Garcia, D L Tierney, S Chong, K Clark, J E Penner-Hahn
X-ray absorption spectroscopy of the zinc site in tRNA-guanine transglycosylase from Escherichia coli.
Biochemistry: 1996, 35(9);3133-9
[PubMed:8608154]
[WorldCat.org]
[DOI]
(P p)
A W Curnow, G A Garcia
tRNA-guanine transglycosylase from Escherichia coli. Minimal tRNA structure and sequence requirements for recognition.
J Biol Chem: 1995, 270(29);17264-7
[PubMed:7615526]
[WorldCat.org]
[DOI]
(P p)
S Chong, A W Curnow, T J Huston, G A Garcia
tRNA-guanine transglycosylase from Escherichia coli is a zinc metalloprotein. Site-directed mutagenesis studies to identify the zinc ligands.
Biochemistry: 1995, 34(11);3694-701
[PubMed:7893665]
[WorldCat.org]
[DOI]
(P p)
S O Mueller, R K Slany
Structural analysis of the interaction of the tRNA modifying enzymes Tgt and QueA with a substrate tRNA.
FEBS Lett: 1995, 361(2-3);259-64
[PubMed:7698334]
[WorldCat.org]
[DOI]
(P p)
A W Curnow, F L Kung, K A Koch, G A Garcia
tRNA-guanine transglycosylase from Escherichia coli: gross tRNA structural requirements for recognition.
Biochemistry: 1993, 32(19);5239-46
[PubMed:8494901]
[WorldCat.org]
[DOI]
(P p)
S Noguchi, Y Nishimura, Y Hirota, S Nishimura
Isolation and characterization of an Escherichia coli mutant lacking tRNA-guanine transglycosylase. Function and biosynthesis of queuosine in tRNA.
J Biol Chem: 1982, 257(11);6544-50
[PubMed:6804468]
[WorldCat.org]
(P p)