RsbR
- Description: activator of RsbT kinase activity, stressosome sensor protein
| Gene name | rsbR |
| Synonyms | ycxR, rsbRA |
| Essential | no |
| Product | activator of RsbT kinase activity, stressosome sensor protein |
| Function | control of SigB activity |
| Gene expression levels in SubtiExpress: rsbR | |
| Interactions involving this protein in SubtInteract: RbsR | |
| Metabolic function and regulation of this protein in SubtiPathways: rsbR | |
| MW, pI | 30 kDa, 4.731 |
| Gene length, protein length | 822 bp, 274 aa |
| Immediate neighbours | ndoA, rsbS |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 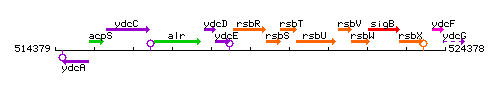
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed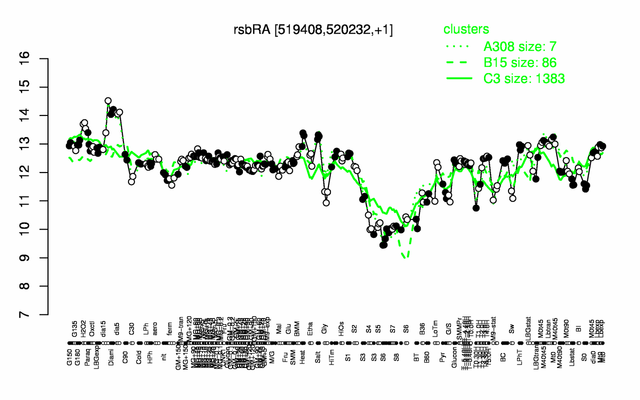
| |
Contents
Categories containing this gene/protein
sigma factors and their control, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU04670
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
Extended information on the protein
- Kinetic information:
- Domains:
- RsbRA is composed of an N-terminal nonheme globin domain and a highly conserved C-terminal STAS (Sulphate Transporter and AntiSigma factor antagonist) domain. The C-terminal STAS domain is the target of the serine/threonine-specific kinase RsbT (see below).
- Effectors of protein activity:
- component of the stressosome
Database entries
- UniProt: P42409
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation: constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Bill Haldenwang, San Antonio, USA
- Chet Price, Davis, USA homepage
- Rick Lewis, Newcastle, UK homepage
Your additional remarks
References
Reviews
Original Articles