FabI
- Description: enoyl-acyl carrier protein reductase
| Gene name | fabI |
| Synonyms | yjbW |
| Essential | no |
| Product | enoyl-acyl carrier protein reductase |
| Function | fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: fabI | |
| Metabolic function and regulation of this protein in SubtiPathways: fabI | |
| MW, pI | 27 kDa, 5.605 |
| Gene length, protein length | 774 bp, 258 aa |
| Immediate neighbours | thiD, cotO |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 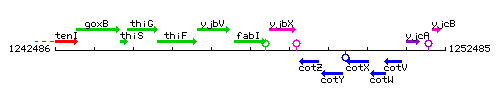
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed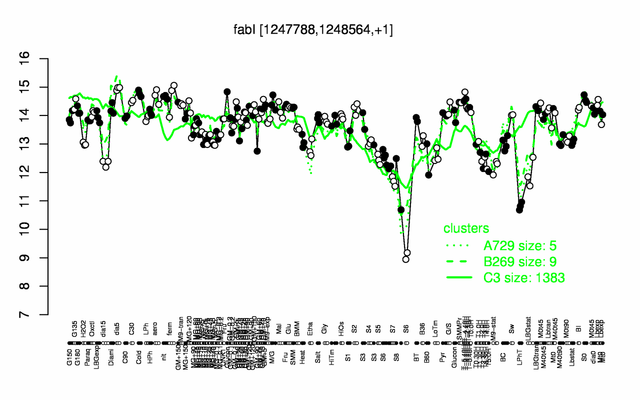
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU11720
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Acyl-[acyl-carrier-protein] + NAD+ = trans-2,3-dehydroacyl-[acyl-carrier-protein] + NADH (according to Swiss-Prot)
- Protein family: FabI subfamily (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- inhibited by triclosan PubMed
Database entries
- UniProt: P54616
- KEGG entry: [3]
- E.C. number: 1.3.1.9
Additional information
Expression and regulation
- Regulation:
- Additional information:
- belongs to the 100 most abundant proteins PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Yasutaro Fujita, Hiroshi Matsuoka, Kazutake Hirooka
Regulation of fatty acid metabolism in bacteria.
Mol Microbiol: 2007, 66(4);829-39
[PubMed:17919287]
[WorldCat.org]
[DOI]
(P p)
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications