GapB
Revision as of 11:25, 7 January 2014 by 134.76.70.252 (talk)
- Description: glyceraldehyde-3-phosphate dehydrogenase, NADP-dependent, gluconeogenic enzyme, forms a transhydrogenation cycle with GapA for balancing of NADPH
| Gene name | gapB |
| Synonyms | ppc |
| Essential | no |
| Product | glyceraldehyde-3-phosphate dehydrogenase 2 |
| Function | anabolic enzyme in gluconeogenesis |
| Gene expression levels in SubtiExpress: gapB | |
| Metabolic function and regulation of this protein in SubtiPathways: gapB | |
| MW, pI | 37,3 kDa, 6.47 |
| Gene length, protein length | 1020 bp, 340 amino acids |
| Immediate neighbours | speD, ytcD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 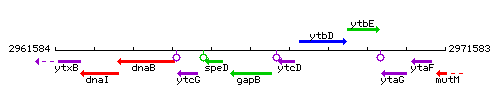
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed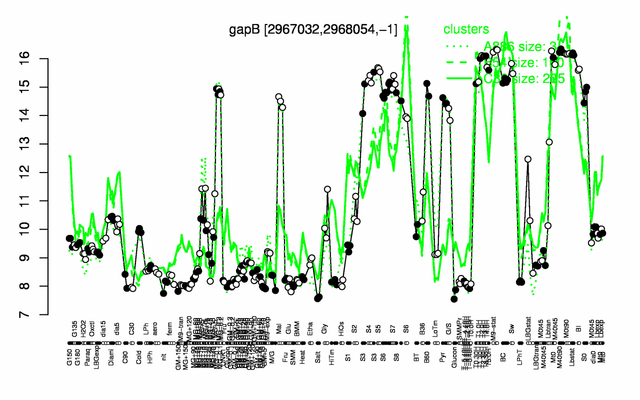
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU29020
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: D-glyceraldehyde 3-phosphate + phosphate + NAD(P)+ = 3-phospho-D-glyceroyl phosphate + NAD(P)H (according to Swiss-Prot)
- This reaction is part of the gluconeogenesis
- Protein family: glyceraldehyde-3-phosphate dehydrogenase family (according to Swiss-Prot)
- Paralogous protein(s): GapA
Extended information on the protein
- Kinetic information: Michaelis-Menten PubMed
- Domains:
- Nucleotid bindinge domain (12-13)
- 2x Glyceraldehyde 3-phosphate binding domain (151-153) & (210-211)
- Modification:
- Cofactor(s): NADP+ (preferentially) and NAD+ PubMed
- Effectors of protein activity:
- Localization: Cytoplasm (Homogeneous) PubMed
Database entries
- Structure: 3PRL (from B. halodurans)
- UniProt: O34425
- KEGG entry: [3]
- E.C. number: 1.2.1.59
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Expression vector:
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Stephane Aymerich, Microbiology and Molecular Genetics, INRA Paris-Grignon, France
Your additional remarks
References