ProA
- Description: glutamate-5-semialdehyde dehydrogenase, required for normal and osmoadaptive proline biosynthesis
| Gene name | proA |
| Synonyms | |
| Essential | no |
| Product | glutamate-5-semialdehyde dehydrogenase |
| Function | biosynthesis of proline |
| Gene expression levels in SubtiExpress: proA | |
| Metabolic function and regulation of this protein in SubtiPathways: proA | |
| MW, pI | 45 kDa, 5.098 |
| Gene length, protein length | 1245 bp, 415 aa |
| Immediate neighbours | proB, ohrA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 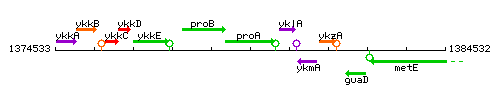
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, coping with hyper-osmotic stress
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13130
Phenotypes of a mutant
- auxotrophic for proline PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-glutamate 5-semialdehyde + phosphate + NADP+ = L-glutamyl 5-phosphate + NADPH (according to Swiss-Prot)
- Protein family: gamma-glutamyl phosphate reductase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- UniProt: P39821
- KEGG entry: [2]
- E.C. number: 1.2.1.41
Additional information
Expression and regulation
- Regulatory mechanism:
- T-box: RNA switch, transcriptional antitermination PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Adrienne Zaprasis, Tamara Hoffmann, Guido Wünsche, Lope A Flórez, Jörg Stülke, Erhard Bremer
Mutational activation of the RocR activator and of a cryptic rocDEF promoter bypass loss of the initial steps of proline biosynthesis in Bacillus subtilis.
Environ Microbiol: 2014, 16(3);701-17
[PubMed:23869754]
[WorldCat.org]
[DOI]
(I p)
Vivianne J Goosens, Ruben A T Mars, Michiel Akeroyd, Andre Vente, Annette Dreisbach, Emma L Denham, Thijs R H M Kouwen, Tjeerd van Rij, Maurien Olsthoorn, Jan Maarten van Dijl
Is proteomics a reliable tool to probe the oxidative folding of bacterial membrane proteins?
Antioxid Redox Signal: 2013, 18(10);1159-64
[PubMed:22540663]
[WorldCat.org]
[DOI]
(I p)
Jeanette Brill, Tamara Hoffmann, Monika Bleisteiner, Erhard Bremer
Osmotically controlled synthesis of the compatible solute proline is critical for cellular defense of Bacillus subtilis against high osmolarity.
J Bacteriol: 2011, 193(19);5335-46
[PubMed:21784929]
[WorldCat.org]
[DOI]
(I p)
Jeanette Brill, Tamara Hoffmann, Harald Putzer, Erhard Bremer
T-box-mediated control of the anabolic proline biosynthetic genes of Bacillus subtilis.
Microbiology (Reading): 2011, 157(Pt 4);977-987
[PubMed:21233158]
[WorldCat.org]
[DOI]
(I p)
Ana Gutiérrez-Preciado, Tina M Henkin, Frank J Grundy, Charles Yanofsky, Enrique Merino
Biochemical features and functional implications of the RNA-based T-box regulatory mechanism.
Microbiol Mol Biol Rev: 2009, 73(1);36-61
[PubMed:19258532]
[WorldCat.org]
[DOI]
(I p)
Mingqing Chen, Hongbo Wei, JunWei Cao, Ruijie Liu, Youliang Wang, Congyi Zheng
Expression of Bacillus subtilis proBA genes and reduction of feedback inhibition of proline synthesis increases proline production and confers osmotolerance in transgenic Arabidopsis.
J Biochem Mol Biol: 2007, 40(3);396-403
[PubMed:17562291]
[WorldCat.org]
[DOI]
(P p)