AbnA
- Description: endo-1,5-alpha-L-arabinosidase
| Gene name | abnA |
| Synonyms | |
| Essential | no |
| Product | endo-1,5-alpha-L-arabinosidase |
| Function | arabinan degradation |
| Gene expression levels in SubtiExpress: abnA | |
| MW, pI | 34 kDa, 8.618 |
| Gene length, protein length | 939 bp, 313 aa |
| Immediate neighbours | araA, ysdC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed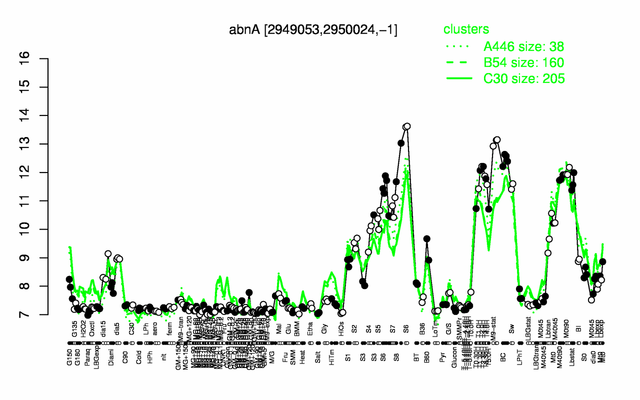
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU28810
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Endohydrolysis of (1->5)-alpha-arabinofuranosidic linkages in (1->5)-arabinans (according to Swiss-Prot)
- Protein family: glycosyl hydrolase 43 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: extracellular (signal peptide) PubMed
Database entries
- Structure: 1UV4
- UniProt: P94522
- KEGG entry: [3]
- E.C. number: 3.2.1.99
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information: the mRNA is very stable (half-life > 15 min) PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Bogumiła C Marciniak, Monika Pabijaniak, Anne de Jong, Robert Dűhring, Gerald Seidel, Wolfgang Hillen, Oscar P Kuipers
High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis.
BMC Genomics: 2012, 13;401
[PubMed:22900538]
[WorldCat.org]
[DOI]
(I e)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Teresa Fontes Leal, Isabel de Sá-Nogueira
Purification, characterization and functional analysis of an endo-arabinanase (AbnA) from Bacillus subtilis.
FEMS Microbiol Lett: 2004, 241(1);41-8
[PubMed:15556708]
[WorldCat.org]
[DOI]
(P p)
Maria Paiva Raposo, José Manuel Inácio, Luís Jaime Mota, Isabel de Sá-Nogueira
Transcriptional regulation of genes encoding arabinan-degrading enzymes in Bacillus subtilis.
J Bacteriol: 2004, 186(5);1287-96
[PubMed:14973026]
[WorldCat.org]
[DOI]
(P p)
G Hambraeus, C von Wachenfeldt, L Hederstedt
Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs.
Mol Genet Genomics: 2003, 269(5);706-14
[PubMed:12884008]
[WorldCat.org]
[DOI]
(P p)