MlpA
- Description: mitochondrial processing peptidase-like, involved in regulation of protease gene expression
| Gene name | mlpA |
| Synonyms | ymxG |
| Essential | no |
| Product | specific processing protease |
| Function | control of proteolyticc activity |
| Gene expression levels in SubtiExpress: mlpA | |
| MW, pI | 45 kDa, 5.275 |
| Gene length, protein length | 1227 bp, 409 aa |
| Immediate neighbours | ylxY, ymxH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 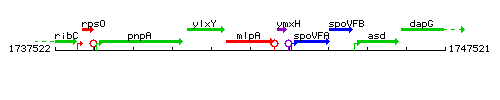
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed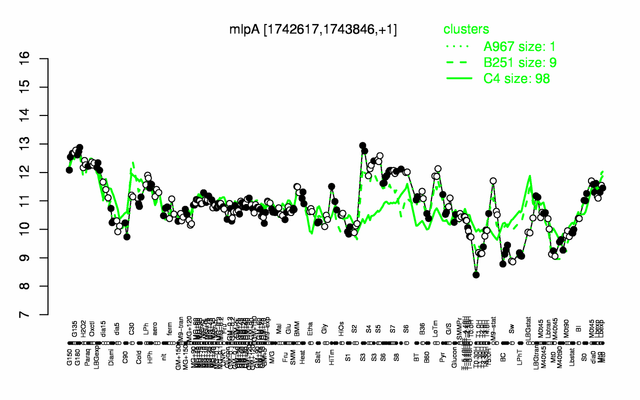
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16710
Phenotypes of a mutant
fivefold increased levels of proteolytic activity in their growth medium PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase M16 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: Q04805
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: deletion mutant, available in van Dijl lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Jan Maarten van Dijl, Groningen, Netherlands
Your additional remarks
References
H Tjalsma, A Bolhuis, J D Jongbloed, S Bron, J M van Dijl
Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome.
Microbiol Mol Biol Rev: 2000, 64(3);515-47
[PubMed:10974125]
[WorldCat.org]
[DOI]
(P p)
A Bolhuis, E Koetje, J Y Dubois, J Vehmaanperä, G Venema, S Bron, J M van Dijl
Did the mitochondrial processing peptidase evolve from a eubacterial regulator of gene expression?
Mol Biol Evol: 2000, 17(1);198-201
[PubMed:10666719]
[WorldCat.org]
[DOI]
(P p)
N Y Chen, S Q Jiang, D A Klein, H Paulus
Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase.
J Biol Chem: 1993, 268(13);9448-65
[PubMed:8098035]
[WorldCat.org]
(P p)